Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration - PubMed (original) (raw)
doi: 10.1007/s00401-007-0237-2. Epub 2007 Jun 20.
Eileen H Bigio, Ian R A Mackenzie, Manuela Neumann, Virginia M-Y Lee, Kimmo J Hatanpaa, Charles L White 3rd, Julie A Schneider, Lea Tenenholz Grinberg, Glenda Halliday, Charles Duyckaerts, James S Lowe, Ida E Holm, Markus Tolnay, Koichi Okamoto, Hideaki Yokoo, Shigeo Murayama, John Woulfe, David G Munoz, Dennis W Dickson, Paul G Ince, John Q Trojanowski, David M A Mann; Consortium for Frontotemporal Lobar Degeneration
Affiliations
- PMID: 17579875
- PMCID: PMC2827877
- DOI: 10.1007/s00401-007-0237-2
Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration
Nigel J Cairns et al. Acta Neuropathol. 2007 Jul.
Abstract
The aim of this study was to improve the neuropathologic recognition and provide criteria for the pathological diagnosis in the neurodegenerative diseases grouped as frontotemporal lobar degeneration (FTLD); revised criteria are proposed. Recent advances in molecular genetics, biochemistry, and neuropathology of FTLD prompted the Midwest Consortium for Frontotemporal Lobar Degeneration and experts at other centers to review and revise the existing neuropathologic diagnostic criteria for FTLD. The proposed criteria for FTLD are based on existing criteria, which include the tauopathies [FTLD with Pick bodies, corticobasal degeneration, progressive supranuclear palsy, sporadic multiple system tauopathy with dementia, argyrophilic grain disease, neurofibrillary tangle dementia, and FTD with microtubule-associated tau (MAPT) gene mutation, also called FTD with parkinsonism linked to chromosome 17 (FTDP-17)]. The proposed criteria take into account new disease entities and include the novel molecular pathology, TDP-43 proteinopathy, now recognized to be the most frequent histological finding in FTLD. TDP-43 is a major component of the pathologic inclusions of most sporadic and familial cases of FTLD with ubiquitin-positive, tau-negative inclusions (FTLD-U) with or without motor neuron disease (MND). Molecular genetic studies of familial cases of FTLD-U have shown that mutations in the progranulin (PGRN) gene are a major genetic cause of FTLD-U. Mutations in valosin-containing protein (VCP) gene are present in rare familial forms of FTD, and some families with FTD and/or MND have been linked to chromosome 9p, and both are types of FTLD-U. Thus, familial TDP-43 proteinopathy is associated with defects in multiple genes, and molecular genetics is required in these cases to correctly identify the causative gene defect. In addition to genetic heterogeneity amongst the TDP-43 proteinopathies, there is also neuropathologic heterogeneity and there is a close relationship between genotype and FTLD-U subtype. In addition to these recent significant advances in the neuropathology of FTLD-U, novel FTLD entities have been further characterized, including neuronal intermediate filament inclusion disease. The proposed criteria incorporate up-to-date neuropathology of FTLD in the light of recent immunohistochemical, biochemical, and genetic advances. These criteria will be of value to the practicing neuropathologist and provide a foundation for clinical, clinico-pathologic, mechanistic studies and in vivo models of pathogenesis of FTLD.
Figures
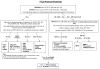
Fig. 1
Frontotemporal lobar degeneration neuropathology algorithm flow chart. AD Alzheimer's disease, AGD argyrophilic grain disease, AMYG amygdala, BIBD basophilic inclusion body disease, CBD corticobasal degeneration, CBLM cerebellum including the dentate nucleus (DN), CHMP2B charged multivesicular body protein 2B gene, CG cingulate gyrus, DLB dementia with Lewy bodies, DLDH dementia lacking distinctive histologic features, also called FTLD according to McKhann et al. [4] criteria, FTLD frontotemporal lobar degeneration, FTLD-U FTLD with ubiquitin-positive, tau-negative inclusions, GP globus pallidus, H&E hematoxylin and eosin, HIP hippocampus, IHC immunohistochemistry, _INAα_-internexin, MAPT microtubule-associated protein tau gene, MED medulla oblongata, MFG middle frontal gyrus, MID midbrain including the substantia nigra, MND motor neuron disease, MSTD sporadic multiple system tauopathy with dementia, NIFID neuronal intermediate filament inclusion disease, NF neurofilament; neurofibrillary tangle dementia, also called tangle predominant form of senile dementia, NOS not otherwise specified, OL occipital lobe, PGRN progranulin gene, FL frontal lobe, PL parietal lobe, PSP progressive supranuclear palsy, SC spinal cord, STG superior temporal gyrus, STR striatum, TDP-43 TAR DNA-binding protein 43, THAL/SUBTN thalamus and subthalamic nucleus, Ub ubiquitin, VCP valosin-containing protein gene, 3R, 4R, or 3R and 4R tau isoforms containing 3, 4, or 3 and 4 microtubule-binding repeats
Fig. 2
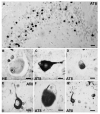
Frontotemporal lobar degeneration with Pick bodies. Pick bodies (arrowheads) and a neurofibrillary tangle (arrow) in the subiculum (a) are immunolabeled by anti-phosphorylated tau antibodies (PHF1 immunohistochemistry). Pick bodies are not immunolabeled with anti-4R tau antibodies (arrowheads), while neurofibrillary tangles are immunolabeled (arrows) (b). Anti-3R tau antibodies clearly label Pick bodies (arrowheads) (c). b 4R tau (ET3) and c 3R tau (RD3) immunohistochemistry. Bars 10 μm
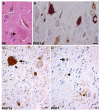
Fig. 3
Corticobasal degeneration. a A swollen achromatic neuron (arrow) in the middle frontal gyrus. Hematoxylin and eosin (HE). b Tau-positive neurofibrillary tangles in the pyramidal neurons of the CA1 hippocampal subfield. c A globose neurofibrillary tangle (arrow) in the locus coeruleus. d An astrocytic plaque (asterisk), coiled body (arrow), and threads (arrowhead) in the deep cortical laminae and white matter of the parietal lobe. b, c, d Anti-phosphorylated tau (PHF1) immunohistochemistry. Bars 10 μm
Fig. 4
Progressive supranuclear palsy. Neurofibrillary tangles in the subthalamic nucleus (a), occulomotor nucleus (b), and locus coeruleus (e). Tufted astrocytes in the putamen (c and d). a, b, c, e Anti-phosphorylated tau (PHF1) immunohistochemistry. d Gallyas silver impregnation. Scale bars a 50 μm, b, c, d, e 10 μm
Fig. 5
Argyrophilic grain disease. A swollen achromatic neuron (arrow) with pale center and more intense tau-immunoreactive periphery in the subiculum. Tau-immunoreactive grains in the neuropil and diffusely stained pyramidal neurons (arrow) indicating a preneurofibrillary tangle stage in the pyramidal layer of the hippocampus (b). A tau-immunoreactive astrocytic inclusion (arrowhead) and oligodendroglial cytoplasmic inclusions called coiled bodies (arrows) in the CA1 subfield of the hippocampus. (a, b, c) Anti-phosphorylated tau (PHF1) immunohistochemistry. Scale bars (a) 100 μm and (b and c) 50 μm
Fig. 6
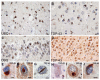
Sporadic multiple system tauopathy with dementia. Neuronal and glial globular inclusions at the gray/white junction. Anti-phosphorylated tau (PHF1) immunohistochemistry. Scale bar 100 μm
Fig. 7
Neurofibrillary tangle dementia. a, b Numerous neurofibrillary tangles in the upper and lower pyramidal neurons of the occipitotemporal cortex; no neuritic plaques or amyloid deposits are present. a Gallyas silver impregnation. b Anti-phosphorylated tau (PHF1) immunohistochemistry. Scale bars 50 μm
Fig. 8
Frontotemporal lobar degeneration (FTLD) with MAPT mutation. Inclusions in FTLD with MAPT G389R mutation (a) and FTLD with MAPT intron 10 + 16 mutation (b–g). a Numerous tau-immuno-reactive Pick body-like inclusions in the granule neurons of the dentate fascia. b A swollen achromatic neuron in the superior temporal gyrus. c A swollen neuron with a central area of pale anti-tau immunoreactivity surrounded by more intense staining. Fibrillary material surrounds the nucleus and extends into the apical dendrite. d An intraneuronal inclusion resembling a Pick body in the superior frontal gyrus. e A neurofibrillary tangle-like inclusion in layer V of the superior frontal gyrus. f A globose neurofibrillary tangle-like inclusion in the dorsal raphé nucleus. g An astrocytic fibrillary inclusion (a) and a coiled body (b) in an oligodendrocyte in the white matter of the frontal lobe. b Hematoxylin and eosin (HE); (a, c–g) anti-phosphorylated tau (AT8) immunohistochemistry. Scale bars 10 μm. (Adapted from Ref. [44]; reproduced with permission)
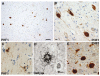
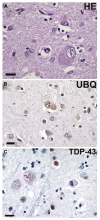
Fig. 9
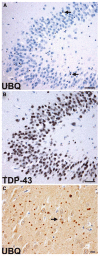
Frontotemporal lobar degeneration (FTLD)-U with or without MND: spectrum of TDP-43 pathology. Adjacent sections of superficial frontal neocortex showing neuronal cytoplasmic inclusions (NCIs), dystrophic neurites (DNs), and isolated neuronal intranuclear inclusions (NIIs) stain for both ubiquitin (a) and TDP-43 (b). NCI in the dentate granule cells stain for ubiquitin (c) and TDP-43 (d). Neuronal and glial inclusions include NCI (e), round and lentiform NIIs (f, g); skein-like (h), and compact round (i) NCI in the lower motor neurons; and glial cytoplasmic inclusion (GCI) (j). (a and c) ubiquitin immunohistochemisty (b, d, e–j TDP-43 immunohistochemistry). Scale bars 10 μm (a–d); 5 μm (e–j) (Adapted from Ref. [16]; reproduced with permission)
Fig. 10
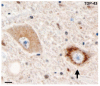
Frontotemporal lobar degeneration-U with MND. Diffuse perinuclear staining in a motor neuron (arrow). TDP-43 immunohistochemistry. Scale bar 10 μm
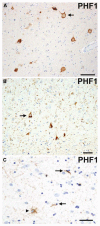
Fig. 11
Frontotemporal lobar degeneration-U, subtypes 1–4. a–d Type 1 is characterized by long and tortuous dystrophic neurites (DNs) in lamiae II/III with relatively few neuronal cytoplasmic inclusions (NCIs) and no neuronal intranuclear inclusion (NII). b Type 2 has numerous NCIs, relatively few DNs, and no NII is present. c Type 3 has numerous NCIs and DNs and an occasional NII in lamina II. d Type 4 pathology in a case of FTD with VCP mutation is characterized by numerous NII and DN, but few NCI. TDP-43 immunohistochemistry. Scale bar 10 μm (a–d). (Adapted from Ref. [14]; reproduced with permission)
Fig. 12
Frontotemporal lobar degeneration with CHMP2B mutation. a Sparse ubiquitin-immunoreactive NCIs (arrows) in the granule neurons of the dentate fascia. The NCIs are not labeled with anti-TDP-43 antibodies (b). Ubiquitin-positive neuropil aggregates and a sparse NCI (arrow) in the frontal lobe of an affected 61-year-old female (c). Scale bars a, b 50 μm, c 10 μm
Fig. 13
Basophilic inclusion body disease. a A basophilic inclusion (BI) in the precentral gyrus (motor cortex), with a similar, weakly ubiquitin-immunoreactive inclusion in (b). c Neurons with basophilic inclusions showing fine granular perikaryal TDP-43 positivity in neurons with BIs on the left and negative in neurons with BIs on the right. a Hematoxylin and eosin; b ubiquitin, and c TDP-43 immunohisto-chemistry. Scale bars 20 μm
Fig. 14
Neuronal intermediate filament inclusion disease. a Eosinophilic Lewy body-like NCI in a pyramidal neuron of the CA1 subfield of the hippocampus. b _α_-Internexin immunoreactive NCIs in layer III of the superior temporal gyrus. c Numerous _α_-internexin immunoreactive NCIs in the granule neurons of the dentate fascia. a Hematoxylin and eosin; b, c _α_-internexin immunohistochemistry. Scale bars 10 μm
Similar articles
- Frontotemporal dementias: update on recent developments in molecular genetics and neuropathology.
Liscić RM. Liscić RM. Arh Hig Rada Toksikol. 2009 Mar;60(1):117-22. doi: 10.2478/10004-1254-60-2009-1921. Arh Hig Rada Toksikol. 2009. PMID: 19329383 Review. - TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions.
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. Cairns NJ, et al. Am J Pathol. 2007 Jul;171(1):227-40. doi: 10.2353/ajpath.2007.070182. Am J Pathol. 2007. PMID: 17591968 Free PMC article. - Clinicopathological and genetic correlates of frontotemporal lobar degeneration and corticobasal degeneration.
Lladó A, Sánchez-Valle R, Rey MJ, Ezquerra M, Tolosa E, Ferrer I, Molinuevo JL; Catalan collaborative Study Group for FTLD. Lladó A, et al. J Neurol. 2008 Apr;255(4):488-94. doi: 10.1007/s00415-008-0565-8. Epub 2008 Mar 25. J Neurol. 2008. PMID: 18357425 - Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation.
Shi J, Shaw CL, Du Plessis D, Richardson AM, Bailey KL, Julien C, Stopford C, Thompson J, Varma A, Craufurd D, Tian J, Pickering-Brown S, Neary D, Snowden JS, Mann DM. Shi J, et al. Acta Neuropathol. 2005 Nov;110(5):501-12. doi: 10.1007/s00401-005-1079-4. Epub 2005 Oct 13. Acta Neuropathol. 2005. PMID: 16222525 - [Frontotemporal dementia (FTD) and genetic mutations including progranulin gene].
Arai T, Hasegawa M, Nishihara M, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Arai T, et al. Rinsho Shinkeigaku. 2008 Nov;48(11):990-3. doi: 10.5692/clinicalneurol.48.990. Rinsho Shinkeigaku. 2008. PMID: 19198141 Review. Japanese.
Cited by
- Language, executive function and social cognition in the diagnosis of frontotemporal dementia syndromes.
Harciarek M, Cosentino S. Harciarek M, et al. Int Rev Psychiatry. 2013 Apr;25(2):178-96. doi: 10.3109/09540261.2013.763340. Int Rev Psychiatry. 2013. PMID: 23611348 Free PMC article. Review. - Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration.
Robinson JL, Porta S, Garrett FG, Zhang P, Xie SX, Suh E, Van Deerlin VM, Abner EL, Jicha GA, Barber JM, Lee VM, Lee EB, Trojanowski JQ, Nelson PT. Robinson JL, et al. Brain. 2020 Sep 1;143(9):2844-2857. doi: 10.1093/brain/awaa219. Brain. 2020. PMID: 32830216 Free PMC article. - Frontotemporal dementia in eight Chinese individuals.
Chao SZ, Rosen HJ, Azor V, Ong H, Tse MM, Lai NB, Hou CE, Seeley WW, Miller BL, Matthews BR. Chao SZ, et al. Neurocase. 2013;19(1):76-84. doi: 10.1080/13554794.2011.654218. Epub 2012 Jun 14. Neurocase. 2013. PMID: 23311888 Free PMC article. - The TMEM106B locus and TDP-43 pathology in older persons without FTLD.
Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA. Yu L, et al. Neurology. 2015 Mar 3;84(9):927-34. doi: 10.1212/WNL.0000000000001313. Epub 2015 Feb 4. Neurology. 2015. PMID: 25653292 Free PMC article. - Degeneration of the locus coeruleus is a common feature of tauopathies and distinct from TDP-43 proteinopathies in the frontotemporal lobar degeneration spectrum.
Ohm DT, Peterson C, Lobrovich R, Cousins KAQ, Gibbons GS, McMillan CT, Wolk DA, Van Deerlin V, Elman L, Spindler M, Deik A, Siderowf A, Trojanowski JQ, Lee EB, Grossman M, Irwin DJ. Ohm DT, et al. Acta Neuropathol. 2020 Nov;140(5):675-693. doi: 10.1007/s00401-020-02210-1. Epub 2020 Aug 17. Acta Neuropathol. 2020. PMID: 32804255 Free PMC article.
References
- Alafuzoff I, Pikkarainen M, Al Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Hauw JJ, Kamphorst W, King A, Kopp N, Korkolopoulou P, Kovacs GG, Meyronet D, Parchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H. Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2006;65:740–757. - PubMed
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
- Behrens MI, Mukherjee O, Tu PH, Liscic RM, Grinberg LT, Carter D, Paulsmeyer K, Taylor-Reinwald L, Gitcho M, Norton JB, Chakraverty S, Goate AM, Morris JC, Cairns NJ. Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alzheimer Dis Assoc Disord. 2007;21:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057105-04/NS/NINDS NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P01-AG03991/AG/NIA NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- AG10124/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- P30 AG013854-14/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- U01 AG016976-09/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- U01-AG16976/AG/NIA NIH HHS/United States
- AG17586/AG/NIA NIH HHS/United States
- P30-AG13854/AG/NIA NIH HHS/United States
- P30-NS057105/NS/NINDS NIH HHS/United States
- P50-AG05681/AG/NIA NIH HHS/United States
- P01 AG003991-26/AG/NIA NIH HHS/United States
- P50 AG005681-25/AG/NIA NIH HHS/United States
- P30 AG010124-16/AG/NIA NIH HHS/United States
- P01 AG017586-09/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous