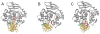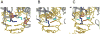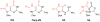Base-excision repair of oxidative DNA damage - PubMed (original) (raw)
Review
Base-excision repair of oxidative DNA damage
Sheila S David et al. Nature. 2007.
Abstract
Maintaining the chemical integrity of DNA in the face of assault by oxidizing agents is a constant challenge for living organisms. Base-excision repair has an important role in preventing mutations associated with a common product of oxidative damage to DNA, 8-oxoguanine. Recent structural studies have shown that 8-oxoguanine DNA glycosylases use an intricate series of steps to locate and excise 8-oxoguanine lesions efficiently against a high background of undamaged bases. The importance of preventing mutations associated with 8-oxoguanine is shown by a direct association between defects in the DNA glycosylase MUTYH and colorectal cancer. The properties of other guanine oxidation products and the associated DNA glycosylases that remove them are now also being revealed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
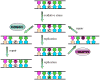
Figure 1. Base Excision Repair (BER) Pathway
Short-patch BER pathway is illustrated in its minimal form. A damage-specific glycosylase removes a damaged base to produce an apurinic-apyrimidinic (AP) site within DNA. Further processing by an AP endonuclease and a phosphoribosyl lyase create the proper ends for a DNA polymerase. Using the opposite strand as a guide, the repair polymerase installs the appropriate nucleotide. DNA ligase seals the backbone. Note: many DNA glycosylases (such as hOGG1) harbor an additional AP endonuclease stand scission activity.
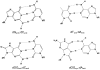
Figure 2. Structures of Base-pairs containing 8-Oxoguanine
The subtle change of placing an oxo-group at C8 and an NH at N7 of G to produce 8-oxoguanine (OG) allows for facile base-pairing with both C and A. The structures of the base pairs of OG:C and OG:A are compared to G:C and T:A
Figure 3. Base Excision Repair Pathway for 8-Oxoguanine
The presence of OG in DNA causes G:C to T:A transversions as illustrated in the center panel. The human BER glycosylases hOGG1 and MutYH remove OG and A from OG:C and OG:A base pairs, respectively. The corresponding enzymes in bacteria are MutM/Fpg and MutY. MutT and MTH1 are not shown, but play an important role in preventing incorportion of OG by hydrolyzing d(OGTP).
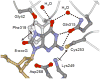
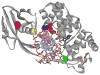
Figure 4. Recognition of OG by hOGG1 observed in the lesion-recognition complexes of hOGG1 with OG:C-containing duplexes
This is a view of the base specific pocket of hOGG1 and shows residues involved in recognizing OG. Importantly, an OG specific hydrogen-bond between NH7 and the Gly 42 carbonyl provides specificity for OG over G. In addition, the dipole associated with the Lys249/Cys 253 pair makes favorable dipole-dipole interactions with OG, but not G.
Figure 5. Comparison of LRC of hOGG1 to nonspecific complexes examining G:C bps observed using the DXL approach
In all structures, the hOGG1 backbone is represented as a dark grey ribbon while the DNA is in gold. Important amino acid side chains are colored fuchsia (Asn or Cys 149), blue (Tyr 203), aqua (Arg 204), purple (Arg 154), orange (Gly 42), light grey (Phe 319), lime (His 270), and pink (Gln 315). Panel A: hOGG1 LRC (with OG:C-containing DNA) where OG is shown in red and the estranged C in green. Panel B: hOGG1 interrogating a G:C bp, with the target G in hot pink and target C in green (Arg 154 is not shown). Panel C: hOGG1 interrogating a G:C bp adjacent to an OG lesion, with OG in red, the target G in hot pink, and the target C in green.
Figure 6. Comparison of LRC of MutM to interrogation complexes with G:C and A:T bps observed using the DXL approach
In all structures, the MutM backbone is represented as a dark grey ribbon while the DNA is in gold. The intercalating Phe 114 is shown in fuchsia, while Arg 112 and Met 77 are in blue and aqua, respectively. Panel A: MutM LRC with OG in red and estranged C in green. Panel B: Interrogation of a G:C bp by MutM, with the target G in red and target C in green. Panel C: Interrogation of an A:T bp by MutM, with the target A in red and target T in green.
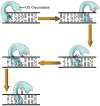
Figure 7. Finding OG lesions- schematic representation of search process based on structures of hOGG1 and MutM trapped complexes
The enzyme tracks rapidly along DNA, inserting a “probe” amino acid residue (shown as a green hexagon) at various base-pairs to test the stability/deformability of the duplex. This results in preferential expulsion of OG from OG:C bps. However, if a G from a G:C bp is extruded, it is captured by an external site and then replaced within the helix. Encountering a G:C bp adjacent to an OG:C bp allows for facile detection of OG since this G cannot be extruded in the same fashion, and will then promote movement of the enzyme to the OG:C bp. At this point, the OG may first be extruded to the exo-site and then is quickly captured in the OG-specific pocket to be clipped-out of the DNA.
Figure 8. Structure of D144N BsMY bound to an OG:A Mismatch-containing duplex highlighting corresponding amino acid variations observed in MAP
The view is down the helix axis with BsMY encircling the DNA. The flipped-out A is shown in black, while the 4Fe-4S cluster of BsMY is shown in orange. Residues in BsMY and corresponding variations in MUTYH are as follows: Y88 (pink), Y165C; G260 (yellow), G382D; S90 (blue), S167F; R91 (red), R168L; P226 (green), P345T; P269 (purple), P391L.
Figure 9. Germline Mutations Observed in MUTYH of MUTYH-associated Polyposis
Shown is an alignment of E. coli MutY, Bacillus stearothermophilus MutY (BsMY) and human MutY homologue (MUTYH). Examples of some of the mutations that have been observed in MAP are indicated. Critical DNA binding motifs are the helix-hairpin-helix (HhH) and the iron-sulfur cluster loop (FCL).,
Figure 10. Guanine oxidation products discussed in this review
A large number of guanine oxidation products have been observed, however, in this review we focus on the hydantoin lesions, guanidinohydantoin (Gh) and spiroiminodihydantoin (Sp). The structures of these lesions resemble OG and FapydG in retaining hydrogen-bonding functionality (red) that can mimic T.
Similar articles
- The role of the N-terminal domain of human apurinic/apyrimidinic endonuclease 1, APE1, in DNA glycosylase stimulation.
Kladova OA, Bazlekowa-Karaban M, Baconnais S, Piétrement O, Ishchenko AA, Matkarimov BT, Iakovlev DA, Vasenko A, Fedorova OS, Le Cam E, Tudek B, Kuznetsov NA, Saparbaev M. Kladova OA, et al. DNA Repair (Amst). 2018 Apr;64:10-25. doi: 10.1016/j.dnarep.2018.02.001. Epub 2018 Feb 11. DNA Repair (Amst). 2018. PMID: 29475157 - The DNA repair enzyme MUTYH potentiates cytotoxicity of the alkylating agent MNNG by interacting with abasic sites.
Raetz AG, Banda DM, Ma X, Xu G, Rajavel AN, McKibbin PL, Lebrilla CB, David SS. Raetz AG, et al. J Biol Chem. 2020 Mar 13;295(11):3692-3707. doi: 10.1074/jbc.RA119.010497. Epub 2020 Jan 30. J Biol Chem. 2020. PMID: 32001618 Free PMC article. - Excision of 8-oxoguanine from methylated CpG dinucleotides by human 8-oxoguanine DNA glycosylase.
Kasymov RD, Grin IR, Endutkin AV, Smirnov SL, Ishchenko AA, Saparbaev MK, Zharkov DO. Kasymov RD, et al. FEBS Lett. 2013 Sep 17;587(18):3129-34. doi: 10.1016/j.febslet.2013.08.008. Epub 2013 Aug 13. FEBS Lett. 2013. PMID: 23954288 - UV-DDB as a General Sensor of DNA Damage in Chromatin: Multifaceted Approaches to Assess Its Direct Role in Base Excision Repair.
Raja SJ, Van Houten B. Raja SJ, et al. Int J Mol Sci. 2023 Jun 15;24(12):10168. doi: 10.3390/ijms241210168. Int J Mol Sci. 2023. PMID: 37373320 Free PMC article. Review. - Repair of 8-oxoG:A mismatches by the MUTYH glycosylase: Mechanism, metals and medicine.
Banda DM, Nuñez NN, Burnside MA, Bradshaw KM, David SS. Banda DM, et al. Free Radic Biol Med. 2017 Jun;107:202-215. doi: 10.1016/j.freeradbiomed.2017.01.008. Epub 2017 Jan 10. Free Radic Biol Med. 2017. PMID: 28087410 Free PMC article. Review.
Cited by
- DNA abasic site-selective enhancement of sanguinarine fluorescence with a large emission shift.
Wu F, Sun Y, Shao Y, Xu S, Liu G, Peng J, Liu L. Wu F, et al. PLoS One. 2012;7(11):e48251. doi: 10.1371/journal.pone.0048251. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185252 Free PMC article. - Base excision repair and cancer.
Wallace SS, Murphy DL, Sweasy JB. Wallace SS, et al. Cancer Lett. 2012 Dec 31;327(1-2):73-89. doi: 10.1016/j.canlet.2011.12.038. Epub 2012 Jan 15. Cancer Lett. 2012. PMID: 22252118 Free PMC article. Review. - Base Excision Repair of N6-Deoxyadenosine Adducts of 1,3-Butadiene.
Wickramaratne S, Banda DM, Ji S, Manlove AH, Malayappan B, Nuñez NN, Samson L, Campbell C, David SS, Tretyakova N. Wickramaratne S, et al. Biochemistry. 2016 Nov 1;55(43):6070-6081. doi: 10.1021/acs.biochem.6b00553. Epub 2016 Oct 21. Biochemistry. 2016. PMID: 27552084 Free PMC article. - Quantification of 8-oxodGuo lesions in double-stranded DNA using a photoelectrochemical DNA sensor.
Zhang B, Guo LH, Greenberg MM. Zhang B, et al. Anal Chem. 2012 Jul 17;84(14):6048-53. doi: 10.1021/ac300866u. Epub 2012 Jun 29. Anal Chem. 2012. PMID: 22746252 Free PMC article.
References
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. - PubMed
- Friedberg EC. DNA Damage and Repair. Nature. 2003;421:436–440. - PubMed
- Pfeifer GP, et al. Tobacoo smoke carcinogens, DNA Damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–51. - PubMed
- Friedberg EC. Inroads into base excision repair II. The discovery of the DNA glycosylases. DNA Repair. 2004;3:1531–1536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008537/GM/NIGMS NIH HHS/United States
- R01 CA090689-07/CA/NCI NIH HHS/United States
- R01 CA067985/CA/NCI NIH HHS/United States
- R01 CA067985-14/CA/NCI NIH HHS/United States
- R01 CA090689/CA/NCI NIH HHS/United States
- T32 CA093247/CA/NCI NIH HHS/United States
- R01 CA067985-13/CA/NCI NIH HHS/United States
- R01 CA090689-08/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials