IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells - PubMed (original) (raw)
. 2007 Jul 26;448(7152):484-487.
doi: 10.1038/nature05970. Epub 2007 Jun 20.
Affiliations
- PMID: 17581588
- PMCID: PMC3805028
- DOI: 10.1038/nature05970
IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells
Thomas Korn et al. Nature. 2007.
Abstract
On activation, naive T cells differentiate into effector T-cell subsets with specific cytokine phenotypes and specialized effector functions. Recently a subset of T cells, distinct from T helper (T(H))1 and T(H)2 cells, producing interleukin (IL)-17 (T(H)17) was defined and seems to have a crucial role in mediating autoimmunity and inducing tissue inflammation. We and others have shown that transforming growth factor (TGF)-beta and IL-6 together induce the differentiation of T(H)17 cells, in which IL-6 has a pivotal function in dictating whether T cells differentiate into Foxp3+ regulatory T cells (T(reg) cells) or T(H)17 cells. Whereas TGF-beta induces Foxp3 and generates T(reg) cells, IL-6 inhibits the generation of T(reg) cells and induces the production of IL-17, suggesting a reciprocal developmental pathway for T(H)17 and T(reg) cells. Here we show that IL-6-deficient (Il6-/-) mice do not develop a T(H)17 response and their peripheral repertoire is dominated by Foxp3+ T(reg) cells. However, deletion of T(reg) cells leads to the reappearance of T(H)17 cells in Il6-/- mice, suggesting an additional pathway by which T(H)17 cells might be generated in vivo. We show that an IL-2 cytokine family member, IL-21, cooperates with TGF-beta to induce T(H)17 cells in naive Il6-/- T cells and that IL-21-receptor-deficient T cells are defective in generating a T(H)17 response.
Figures
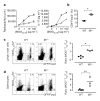
Figure 1. In the absence of IL-6, antigen-specific Foxp31 Treg cells expand at the expense of effector T cells (Teff cells)in vivo
Foxp3gfp.KI (filled circles)or _Il6_−/− × Foxp3gfp.KI (_Il6_−/−; open triangles) mice were immunized with MOG35–55/CFA. a, Draining lymph-node cells were tested for MOG-specific proliferation and IL-17 production (means ± s.d. for triplicate determinations). b, The fraction of Foxp3/GFP+ T cells was determined ex vivo by flow cytometry (asterisk, P < 6 × 10−7; _t_-test). WT, wild type. c, d, Lymphnode cells (c) and splenocytes (d) from MOG35–55/CFA-immunized WT and _Il6_−/− mice were cultured for four days in the presence of MOG35–55 and stained with a MOG35–55/IAb tetramer. The ratios of antigen-specific Treg cells (MOG tetramer+CD4+Foxp3+) to Teff (MOG tetramer+CD4+Foxp3−) cells are presented (asterisk, P < 0.0003; two asterisks, P < 0.05; _t_-test).
Figure 2. Depletion of Treg cells in _Il6_−/− mice restores the development of TH17 cells and susceptibility to EAE
_Il6_−/− × Foxp3gfp.KI mice were treated with antibody against CD25 to deplete Treg cells or with a control immunoglobulin (rat IgG1) and then immunized with MOG35–55/CFA. a, b, Clinical EAE scores (means and s.e.m.) (a) and linear regression analysis in acute (b, top) and chronic (b, bottom) stages of the disease for wild-type (WT), control _Il6_−/− and Treg-depleted Il6_−/− mice (n.s., not significant). c, Lymph-node (LN) cells and mononuclear cells from the central nervous system (CNS) were recovered on days 6, 14–17 (peak disease) and 29 (recovery) and stained for CD4 and intracellular IL-17 and IFN-γ. The numbers in the quadrants show percentages. d, Splenocytes (day 10) were stimulated with MOG35–55_in vitro. Culture supernatants were collected after 48 h, and IL-17 and IFN-γ concentrations were determined. Filled circles, WT (IgG); open triangles, _Il6_−/− (IgG); open circles, _Il6_−/− (anti-CD25).
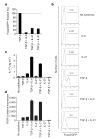
Figure 3. Inhibition of induction of Treg cells and generation of TH17 cells by IL-21
CD4+CD62LhiFoxp3/GFP− T cells from Foxp3gfp.KI mice were stimulated with anti-CD3 and anti-CD28 for three days in the presence of the indicated cytokines. a, The expression of Foxp3/GFP was measured and the fraction of Foxp3+ cells induced by TGF-β was normalized to 100%. b, Individual histograms showing Foxp3/GFP expression. The numbers above the histogram regions (horizontal lines) represent the percentages of Foxp3/GFP+ cells. c, IL-17 production in these cultures after 48 h as measured by ELISA. d, ROR-γt expression as determined by quantitative RT–PCR in naive 2D2 (ref. 30) T cells activated for 48 h with anti-CD3 in the presence of irradiated syngeneic antigen-presenting cells and the indicated cytokines. ROR-γt expression is shown as mean and s.e.m. for duplicate determinations, relative to β-actin.
Figure 4. IL-21-driven TH17 differentiation is independent of IL-6
Naive (a, b), total (c) or CD44+CD4+ T cells (d, e) were cultured with anti-CD3 plus corresponding irradiated antigen-presenting cells (a, c) or anti-CD3 plus anti-CD28 (b, d, e) and the indicated cytokines. a, c, Percentages of IL-17+ and IFN-γ+ cells in T cells from wild-type (WT) or _Il6_−/− mice (a) and WT or _Il21r_−/− mice (c) after four days of culture. b, IL-21 mRNA was determined by quantitative RT–PCR (means and s.e.m. for duplicate determinations). d, e, CD4+CD44+ T cells from WT or _Il21r_−/− mice were stimulated with or without recombinant IL-23 for 48 h. IL-17 and IFN-γ production were determined by intracellular cytokine staining (d) and ELISA (e; open columns, WT; filled columns, _Il21r_−/−) (asterisk, P < 0.0005; two asterisks, P < 0.003; _t_-test). f, WT (filled circles) and _Il21r_−/− (open triangles) mice were immunized with ovalbumin 323–339 peptide (OVA323–339)/CFA. Draining lymph-node cells were assayed for antigen-specific proliferation and cytokine production (means and s.d. for triplicate cultures).
Comment in
- Immunology: narcissistic helpers.
Palmer MT, Weaver CT. Palmer MT, et al. Nature. 2007 Jul 26;448(7152):416-8. doi: 10.1038/448416a. Nature. 2007. PMID: 17653176 No abstract available.
Similar articles
- TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function.
Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. Zhou L, et al. Nature. 2008 May 8;453(7192):236-40. doi: 10.1038/nature06878. Epub 2008 Mar 26. Nature. 2008. PMID: 18368049 Free PMC article. - The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis.
Korn T, Anderson AC, Bettelli E, Oukka M. Korn T, et al. J Neuroimmunol. 2007 Nov;191(1-2):51-60. doi: 10.1016/j.jneuroim.2007.09.009. Epub 2007 Oct 3. J Neuroimmunol. 2007. PMID: 17916388 Free PMC article. Review. - IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells.
Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. Dardalhon V, et al. Nat Immunol. 2008 Dec;9(12):1347-55. doi: 10.1038/ni.1677. Epub 2008 Nov 9. Nat Immunol. 2008. PMID: 18997793 Free PMC article. - Essential autocrine regulation by IL-21 in the generation of inflammatory T cells.
Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Nurieva R, et al. Nature. 2007 Jul 26;448(7152):480-3. doi: 10.1038/nature05969. Epub 2007 Jun 20. Nature. 2007. PMID: 17581589 - IL-17 and Th17 Cells.
Korn T, Bettelli E, Oukka M, Kuchroo VK. Korn T, et al. Annu Rev Immunol. 2009;27:485-517. doi: 10.1146/annurev.immunol.021908.132710. Annu Rev Immunol. 2009. PMID: 19132915 Review.
Cited by
- The Emerging Role of Interleukin-21 in Transplantation.
Xie A, Buras ED, Xia J, Chen W. Xie A, et al. J Clin Cell Immunol. 2012 Aug 24;Suppl 9(2):1-7. doi: 10.4172/2155-9899.S9-002. J Clin Cell Immunol. 2012. PMID: 23828737 Free PMC article. - CD4(+) T-cell subsets and host defense in the lung.
Kolls JK. Kolls JK. Immunol Rev. 2013 Mar;252(1):156-63. doi: 10.1111/imr.12030. Immunol Rev. 2013. PMID: 23405903 Free PMC article. Review. - The effects of TLR activation on T-cell development and differentiation.
Jin B, Sun T, Yu XH, Yang YX, Yeo AE. Jin B, et al. Clin Dev Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. Epub 2012 Jun 7. Clin Dev Immunol. 2012. PMID: 22737174 Free PMC article. Review. - Positive evidence for vitamin A role in prevention of type 1 diabetes.
Yosaee S, Akbari Fakhrabadi M, Shidfar F. Yosaee S, et al. World J Diabetes. 2016 May 10;7(9):177-88. doi: 10.4239/wjd.v7.i9.177. World J Diabetes. 2016. PMID: 27162582 Free PMC article. Review. - Interleukin-17 in Chronic Inflammatory Neurological Diseases.
Milovanovic J, Arsenijevic A, Stojanovic B, Kanjevac T, Arsenijevic D, Radosavljevic G, Milovanovic M, Arsenijevic N. Milovanovic J, et al. Front Immunol. 2020 Jun 3;11:947. doi: 10.3389/fimmu.2020.00947. eCollection 2020. Front Immunol. 2020. PMID: 32582147 Free PMC article. Review.
References
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
- Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. - PubMed
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. - PubMed
- Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nature Med. 2007;13:139–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases