Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron - PubMed (original) (raw)
Comparative Study
Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron
Shin-ya Kawaguchi et al. J Neurosci. 2007.
Abstract
Fast inhibitory synaptic transmission is predominantly mediated by GABA(A) receptor (GABA(A)R) in the CNS. Although several types of neuronal activity-dependent plasticity at GABAergic synapses have been reported, the detailed mechanism is elusive. Here we show that binding of structurally altered GABA(A)R-associated protein (GABARAP) to GABA(A)R gamma2 subunit and to tubulin is critical for long-term potentiation [called rebound potentiation (RP)] at inhibitory synapses on a cerebellar Purkinje neuron (PN). Either inhibition of GABARAP association with GABA(A)Rgamma2 or deletion of tubulin binding region of GABARAP impaired RP. Inhibition of tubulin polymerization also suppressed RP. Thus, precise regulation of GABA(A)Rgamma2-GABARAP-microtubule interaction is critical for RP. Furthermore, competitive inhibition of GABARAP binding to GABA(A)Rgamma2 after the RP establishment attenuated the potentiated response, suggesting that GABARAP is critical not only for the induction but also for the maintenance of RP. Fluorescence resonance energy transfer analysis revealed that GABARAP underwent sustained structural alteration after brief depolarization of a PN depending on the activity of Ca2+/calmodulin-dependent protein kinase II (CaMKII), which is required for the RP induction. The susceptibility of GABARAP to undergo structural alteration was abolished by an amino acid replacement in GABARAP. Furthermore, RP was impaired by expression of the mutant GABARAP with the replacement. Together, we conclude that GABA(A)R association with structurally altered GABARAP downstream of CaMKII activation is essential for RP.
Figures
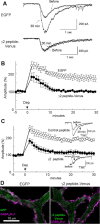
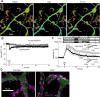
Figure 1.
Involvement of GABARAP in RP. A, B, Representative traces (A) and time courses of amplitudes (B) of current responses to GABA before and after the conditioning depolarization (0 mV for 500 ms, 5 times at 0.5 Hz) recorded from a PN transfected with EGFP or fusion protein of 18 amino acids peptide of GABAAR γ2 subunit and Venus. n = 5 for each. C, Representative traces and time courses of amplitudes of GABA responses before and after the conditioning depolarization with γ2 or control peptide applied through a patch pipette. n = 5 for each. D, Immunocytochemical staining of GABAAR α1 subunit and EGFP transfected into a PN. Dendrites of PNs are shown.
Figure 2.
Requirement of GABARAP association with GABAARγ2 for RP at synapses. A, Representative traces of mIPSCs before and 30 min after the conditioning depolarization in the presence of control peptide or γ2 peptide. B, Cumulative probability histogram of amplitudes of mIPSCs (>400 events for each) before and 30 min after the conditioning depolarization in the presence of control or γ2 peptide. C, Time courses of amplitudes of mIPSCs with γ2 or control peptide applied through a patch pipette. n = 5 for each. Dep, Depolarization. D, Representative traces of presynaptic action potentials and postsynaptic IPSCs before and 20 min after the conditioning depolarization in the presence of control or γ2 peptide. IN, Inhibitory interneuron. Each trace was obtained by averaging 15 events. E, Time courses of amplitudes of evoked IPSCs in the presence of control or γ2 peptide.
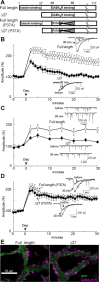
Figure 3.
Binding of GABARAP to tubulin is critical for RP. A, The wild-type and mutant GABARAP proteins. Deletion of N-terminal 27 amino acids abolishes tubulin binding. P37A point mutation reduces the affinity to GABAARγ2 subunit. B, Representative traces and time courses of amplitudes of GABA responses in a PN transfected with either full-length or Δ27 GABARAP. n = 5 for each. C, Representative traces and time courses of amplitudes of mIPSCs in a PN transfected with either full-length (n = 5) or Δ27 GABARAP (n = 8). D, Representative traces and time courses of amplitudes of GABA responses in a PN transfected with either full-length (n = 6) or Δ27 GABARAP (n = 5) containing P37A point mutation. E, Immunocytochemical staining of EGFP and surface GABAAR α1 subunit in a PN transfected with either full-length or Δ27 GABARAP together with EGFP.
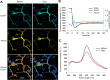
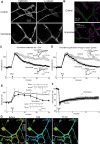
Figure 4.
FRET decrease of CGV probe in response to depolarization of a PN. A, Representative fluorescence images of ECFP and Venus and the ratio images of Venus/ECFP before and immediately after the conditioning depolarization (Dep). B, Time courses of fluorescence intensity of ECFP (blue) and Venus (green) and that of the fluorescence ratio (Venus/ECFP, black). The conditioning depolarization was applied at 0 s. A.U., Arbitrary unit. C, Fluorescence emission spectrum of CGV probe excited by 440 nm laser. Data obtained before (black) and 10 min after the conditioning depolarization (red) are shown.
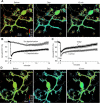
Figure 5.
Long-lasting GABARAP structural alteration detected with FRET. A, Representative pseudocolor fluorescence ratio images (Venus/ECFP) before, during, and 15 min after the conditioning depolarization (Dep) in a PN. B, Time courses of the normalized fluorescence ratio in a proximal dendrite of PNs. The conditioning depolarization was applied at 0 min. n = 13 (Depolarization) and n = 7 (No depolarization). C, Time courses of the normalized fluorescence ratio in the absence or presence of KN62 (n = 8). The control data are same as the depolarization data in B. D, Representative images of the fluorescence ratio of CGV probe before, during, and 15 min after the conditioning depolarization in the presence of KN62.
Figure 6.
A critical role of GABARAP conformation change in RP. A, Representative ratio images (Venus/ECFP) before, during, and 15 min after the conditioning depolarization (Dep) in a PN transfected with the CGV probe containing V33E mutation. B, Time courses of normalized fluorescent ratio (Venus/ECFP) in a PN transfected with the CGV probe containing V33E mutation. n = 9 (Depolarization) and n = 6 (No depolarization). C, Representative traces and time courses of amplitudes of GABA response before and after the conditioning depolarization in PNs transfected either with the full-length GABARAP containing V33E mutation alone (n = 6) or with both V33E and P37A mutations (n = 5). D, Immunocytochemical images for staining of EGFP and surface GABAAR α1 subunit on the plasma membrane of PN dendrites. Either the full-length GABARAP (Wild type) or V33E GABARAP (V33E) together with EGFP was transfected into a PN.
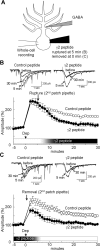
Figure 7.
Binding of GABARAP and GABAARγ2 subunit is required for the maintenance of RP. A, Schematic diagram to show the experimental design. GABARAP binding to GABAARγ2 subunit was inhibited by the γ2 peptide (100 μ
m
) from 5 min after the depolarization (B) or until the end of conditioning depolarization (C). B, C, Representative traces and time courses of amplitudes of GABA responses before and after the conditioning depolarization (Dep) with either the γ2 peptide or the control peptide introduced into a PN from 5 min (B) or until 0.5 min (C) through the second patch pipette. n = 5 for each.
Figure 8.
Involvement of microtubule in RP. A, B, Immunocytochemical staining of calbindin and α-tubulin (A) or GABAARα1 (B) in dendrites of PNs with or without (control) vincristine treatment. Distribution of α-tubulin was changed by the vincristine treatment from filamentous distribution along dendritic shafts to irregular distribution consisting of sparse and accumulated regions (yellow arrowheads). Despite microtubule alteration, GABAAR distribution was not clearly affected by the treatment. C, D, Representative traces and time courses of amplitudes of GABA responses before and after the conditioning depolarization (Dep) in a PN. C, Neurons were treated with vincristine for longer than 2 h. n = 6 (Vincristine) and n = 5 (Control). D, Vincristine was applied through a patch pipette. E, Representative traces and time courses of amplitudes of mIPSCs in a PN to which vincristine was applied through a patch pipette. n = 5 (Vincristine) and n = 6 (Control). F, Time courses of the normalized fluorescence ratio (Venus/ECFP) in the presence (Vincristine, n = 7) or absence (Control, n = 13) of vincristine applied through a patch pipette. G, Fluorescence intensity ratio images (Venus/ECFP) before, during, and 15 min after the conditioning depolarization in the presence of vincristine in the patch pipette.
Similar articles
- Signaling cascade regulating long-term potentiation of GABA(A) receptor responsiveness in cerebellar Purkinje neurons.
Kawaguchi SY, Hirano T. Kawaguchi SY, et al. J Neurosci. 2002 May 15;22(10):3969-76. doi: 10.1523/JNEUROSCI.22-10-03969.2002. J Neurosci. 2002. PMID: 12019316 Free PMC article. - mGluR1-mediated facilitation of long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron.
Sugiyama Y, Kawaguchi SY, Hirano T. Sugiyama Y, et al. Eur J Neurosci. 2008 Feb;27(4):884-96. doi: 10.1111/j.1460-9568.2008.06063.x. Epub 2008 Feb 13. Eur J Neurosci. 2008. PMID: 18279362 - Integrin alpha3beta1 suppresses long-term potentiation at inhibitory synapses on the cerebellar Purkinje neuron.
Kawaguchi SY, Hirano T. Kawaguchi SY, et al. Mol Cell Neurosci. 2006 Mar;31(3):416-26. doi: 10.1016/j.mcn.2005.10.012. Epub 2005 Nov 22. Mol Cell Neurosci. 2006. PMID: 16307893 - Regulation and functional roles of rebound potentiation at cerebellar stellate cell-Purkinje cell synapses.
Hirano T, Kawaguchi SY. Hirano T, et al. Front Cell Neurosci. 2014 Feb 18;8:42. doi: 10.3389/fncel.2014.00042. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24600347 Free PMC article. Review. - GABARAP: lessons for synaptogenesis.
Coyle JE, Nikolov DB. Coyle JE, et al. Neuroscientist. 2003 Jun;9(3):205-16. doi: 10.1177/1073858403009003013. Neuroscientist. 2003. PMID: 15065816 Review.
Cited by
- Facial stimulation induces long-term depression at cerebellar molecular layer interneuron-Purkinje cell synapses in vivo in mice.
Bing YH, Wu MC, Chu CP, Qiu DL. Bing YH, et al. Front Cell Neurosci. 2015 Jun 9;9:214. doi: 10.3389/fncel.2015.00214. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26106296 Free PMC article. - Analogue signaling of somatodendritic synaptic activity to axon enhances GABA release in young cerebellar molecular layer interneurons.
Trigo F, Kawaguchi SY. Trigo F, et al. Elife. 2023 Aug 11;12:e85971. doi: 10.7554/eLife.85971. Elife. 2023. PMID: 37565643 Free PMC article. - An efficient method for the long-term and specific expression of exogenous cDNAs in cultured Purkinje neurons.
Wagner W, McCroskery S, Hammer JA 3rd. Wagner W, et al. J Neurosci Methods. 2011 Sep 15;200(2):95-105. doi: 10.1016/j.jneumeth.2011.06.006. Epub 2011 Jun 25. J Neurosci Methods. 2011. PMID: 21708190 Free PMC article. - Axonal GABAA receptors depolarize presynaptic terminals and facilitate transmitter release in cerebellar Purkinje cells.
Zorrilla de San Martin J, Trigo FF, Kawaguchi SY. Zorrilla de San Martin J, et al. J Physiol. 2017 Dec 15;595(24):7477-7493. doi: 10.1113/JP275369. Epub 2017 Nov 21. J Physiol. 2017. PMID: 29072780 Free PMC article. - LTD, RP, and Motor Learning.
Hirano T, Yamazaki Y, Nakamura Y. Hirano T, et al. Cerebellum. 2016 Feb;15(1):51-53. doi: 10.1007/s12311-015-0698-0. Cerebellum. 2016. PMID: 26160222 Review.
References
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. - PubMed
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. - PubMed
- Chen Z, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous