Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha - PubMed (original) (raw)
Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha
Karen Bozak Jelks et al. J Neurosci. 2007.
Abstract
Estradiol mediates structural changes at synapses of the hippocampus, an area in the brain important for learning and memory. This study was designed to test the hypothesis that estradiol mediates subcellular changes of synaptic proteins to induce new synapses via an estrogen receptor (ER)-mediated process. To elucidate the mechanisms involved in glutamatergic synapse formation, we investigated effects of estradiol on synaptic proteins in cultured hippocampal neurons using immunocytochemistry and confocal microscopy. Synaptic protein distribution and size were identified with antibodies to the presynaptic vesicular glutamate transporter protein (vGlut1) and postsynaptic NMDA receptor (NR1 subunit). We observed an increase in synapse density, as detected by NR1 and vGlut1 colocalization, along dendrites of neurons cultured in steroid-stripped media and exposed to estradiol (10 nM) for 48 h. Additionally, the NR1 subunit was enriched at synaptic clusters. Immunocytochemistry and confocal imaging revealed punctate staining of extranuclear ERs along dendrites of hippocampal neurons expressing NR1. Estradiol increased the density of both ER-alpha and ER-beta protein clusters along dendrites. To test whether ERs play an important functional role in the estradiol-induced synaptogenesis, we used the ER antagonist [7alpha,17beta-[9[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol (ICI 182,780)] and the ER-alpha- and ER-beta-specific agonists [1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT) and 2,3-bis(4-hydroxyphenyl) propionitrile (DPN), respectively]. ICI 182,780 blocked the increase in synapse density. Treatment with PPT, but not DPN, induced significant increases in synapse density that mimicked treatment with estradiol. Together, our results demonstrate that estradiol stimulates glutamatergic synapse formation in the developing hippocampus through an ER-alpha-dependent mechanism. These findings carry profound implications regarding the potential of estrogen to influence learning, memory, and possibly hormone-modulated neurodegeneration.
Figures
Figure 1.
Estradiol (E; 10 n
m
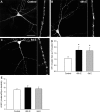
) exposure enhances the cluster size of NMDARs. ICC of NR1 and confocal imaging were used to examine the effect of estradiol exposure on NMDAR clustering and cluster size in hippocampal cultures. A–C, Confocal imaging of NR1 revealed punctate clustering of NMDARs in control cultures (A). A 48 h exposure to estradiol (10 n
m
; B) and prolonged 6 d of exposure to estradiol (10 n
m
; C) resulted in an increase in NR1 cluster size. D, E, Quantification of the effects of estradiol on clustering revealed a significant increase in NR1 cluster size (D) and not in density (E). All values are mean ± SEM. The asterisk indicates a significant difference from control (p < 0.05, Student's t test; n = 23–26). Scale bar, 40 μm.
Figure 2.
Estradiol (E; 10 n
m
) exposure enhances the density and cluster size of vesicular glutamate transporter (vGlut1) proteins. ICC of vGlut1 and confocal imaging were used to examine the effect of estradiol exposure on vGlut1 clustering and cluster size in hippocampal cultures. A–C, Confocal imaging of vGlut1 revealed punctate clustering of vGlut1 in control cultures (A). A 48 h exposure to estradiol (10 n
m
; B) and prolonged 6 d of exposure to estradiol (10 n
m
; C) resulted in an increase in cluster size and density. D, E, Quantification of the effects of estradiol on clustering revealed a significant increase in vGlut1 cluster size (D) and vGlut1 cluster density (E). All values are mean ± SEM. The asterisk indicates a significant difference from control (p < 0.05 Student's t test; n = 23–26). Scale bar, 40 μm.
Figure 3.
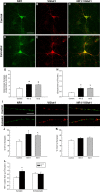
Estradiol treatment increases synaptic cluster density and cluster size. A–F, I, Double-label ICC for NR1 (green) and vGlut1 (red); yellow in the superimposed images indicates colocalization. G, H, Estradiol treatment significantly increased the density of colocalized NR1 and vGlut1 clusters (G) and cluster size (H). *Significant difference from control (48 h: p < 0.0005, n = 24; 6 d: p < 0.003, n = 23; Student's t test). J, Quantification of NR1 at synaptic clusters revealed that estradiol also significantly increases the percentage of NR1 proteins. The asterisk indicates a significant difference from control [48 h, p < 0.002 (n = 24) and p < 0.01 (n = 23); Student's t test]. K, Quantification of vGlut1 at synaptic clusters showed no difference between control and treated hippocampal pyramidal neurons. L, The estradiol-induced increase in NR1 cluster size was limited to the synaptic compartment (p < 0.0005, Student's t test; n = 39) with no changes occurring in extrasynaptic NR1 clusters (p < 0.81, Student's t test; n = 39). All values are mean ± SEM. Scale bars: A–F, 40 μm; I, 10 μm.
Figure 4.
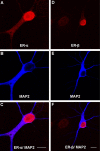
A, B, D, E, Double-label fluorescent ICC for ER-α and MAP2 (A, B) or ER-β and MAP2 (D, E) in cultured hippocampal neurons grown on a bedlayer of astrocytes at 6 d.i.v. C, F, Low-magnification representative micrographs of ER- α/MAP2 and ER-β/MAP2 overlay reveal immunoreactive nuclei and some diffuse ER-α and ER-β cytoplasmic staining in MAP2-positive neurons. Subsequent fluorescent ICC and confocal imaging was used to identify ER-α and ER-β clusters along dendrites of hippocampal neurons cultured on glass coverslips suspended above a bedlayer of astrocytes at 6 d.i.v. Scale bars, 10 μm.
Figure 5.
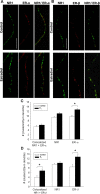
A, Double-label ICC for NR1 (green) and ER-α (red); yellow in the superimposed images indicates colocalization of representative micrographs from control and estradiol-treated cultures of rat hippocampal neurons cultured alone on glass coverslips grown in culture wells suspended above astrocyte bedlayer. ER-α clusters displayed a fine punctuate pattern in control cultures as detected by the C1355 polyclonal antibody. B, Double-label ICC for NR1 (green) and ER-β (red); yellow in the superimposed images indicates colocalization of representative micrographs from cultures of rat hippocampal neurons cultured alone on glass coverslips grown in culture wells suspended above astrocyte bedlayer. ER-β clusters displayed a fine punctuate pattern in control cultures as detected by the Zymed polyclonal antibody. C, Quantification of the average cluster density of NR1, ER-α, and colocalized NR1/ER-α observed in control and estradiol-treated cultures revealed that estradiol treatment significantly increases the density of ER-α clusters (*p < 0.03, Student's t test; n = 33). Comparison of control and estradiol-treated neurons revealed no significant difference in cluster density of NR1 proteins and colocalized NR1/ER-α proteins. D, Quantification of the average cluster density of NR1, ER-β, and colocalized NR1/ER-β observed in control and estradiol-treated cultures revealed that estradiol treatment significantly increases the density of ER-β clusters (*p < 0.003, Student's t test; n = 35) and colocalized NR1/ER-β (*p < 0.007, Student's t test; n = 35). Comparison of control and estradiol-treated neurons revealed no significant difference in cluster density of NR1 proteins. All values are mean ± SEM. The asterisks indicate a significant difference from control. Scale bar, 10 μm. E2, Estradiol.
Figure 6.
A, Estradiol-induced increase in synaptic cluster density is inhibited in neuron cultures pretreated with ICI 182,780 (1 μ
m
), an ER antagonist, before estradiol exposure. Scale bar, 10 μm. B, Quantification of NR1 (green) and vGlut1 (red) colocalization in cultures treated with ICI before estradiol were no different than control levels. Cultures exposed to ICI alone, without any estradiol, were also no different than control levels. *, Significant difference from control; #, significant difference from estradiol and ICI treated; @, significant difference from ICI-only treated (p < 0.01, ANOVA with Newman–Keuls post hoc; n = 18–29). All values are mean ± SEM. E, Estradiol; ICI, ICI 182,780.
Figure 7.
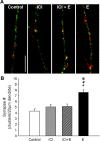
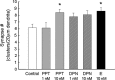
The ER-α agonist PPT (10 n
m
) increases the number of synapses similar to that observed with estradiol (E) in hippocampal neurons undergoing in vitro development. ICC and confocal imaging of colocalized NR1 and vGlut1 proteins were used to determine the effects of the ER-α-specific agonist PPT and ER-β-specific agonist DPN on the density of synaptic clusters along dendrites. *Significant increase above control was found with exposure to estradiol (10 n
m
; 48 h; p < 0.027, ANOVA with Newman–Keuls post hoc; n = 22) and the ER-α-specific agonist PPT (10 n
m
; 48 h; p < 0.034, ANOVA with Newman–Keuls post hoc; n = 20). Comparison of vehicle-treated neurons to PPT-treated (1 n
m
), DPN-treated (1 n
m
), and DPN-treated (10 n
m
) neurons revealed no significant differences in synapse density when treatment groups were analyzed together by ANOVA with Newman–Keuls post hoc comparison. All values are mean ± SEM.
Similar articles
- Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses.
Smith CC, Vedder LC, McMahon LL. Smith CC, et al. Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S130-42. doi: 10.1016/j.psyneuen.2009.06.003. Psychoneuroendocrinology. 2009. PMID: 19596521 Free PMC article. Review. - Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus.
Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Waters EM, et al. Brain Res. 2009 Sep 22;1290:1-11. doi: 10.1016/j.brainres.2009.06.090. Epub 2009 Jul 9. Brain Res. 2009. PMID: 19596275 Free PMC article. - Distribution, density, and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons.
Cottrell JR, Dubé GR, Egles C, Liu G. Cottrell JR, et al. J Neurophysiol. 2000 Sep;84(3):1573-87. doi: 10.1152/jn.2000.84.3.1573. J Neurophysiol. 2000. PMID: 10980028 - Effect of oestrogen receptor alpha and beta agonists on brain N-methyl-D-aspartate receptors.
Morissette M, Le Saux M, Di Paolo T. Morissette M, et al. J Neuroendocrinol. 2008 Aug;20(8):1006-14. doi: 10.1111/j.1365-2826.2008.01754.x. Epub 2008 May 27. J Neuroendocrinol. 2008. PMID: 18510708 - Modulation of synaptic plasticity by brain estrogen in the hippocampus.
Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanaka Y, Ogiue-Ikeda M. Mukai H, et al. Biochim Biophys Acta. 2010 Oct;1800(10):1030-44. doi: 10.1016/j.bbagen.2009.11.002. Epub 2009 Nov 10. Biochim Biophys Acta. 2010. PMID: 19909788 Review.
Cited by
- Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice.
Spencer JL, Waters EM, Milner TA, McEwen BS. Spencer JL, et al. Neuroscience. 2008 Sep 9;155(4):1106-19. doi: 10.1016/j.neuroscience.2008.05.049. Epub 2008 Jun 8. Neuroscience. 2008. PMID: 18601981 Free PMC article. - Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses.
Smith CC, Vedder LC, McMahon LL. Smith CC, et al. Psychoneuroendocrinology. 2009 Dec;34 Suppl 1:S130-42. doi: 10.1016/j.psyneuen.2009.06.003. Psychoneuroendocrinology. 2009. PMID: 19596521 Free PMC article. Review. - Low dietary soy isoflavonoids increase hippocampal spine synapse density in ovariectomized rats.
MacLusky NJ, Thomas G, Leranth C. MacLusky NJ, et al. Brain Res. 2017 Feb 15;1657:361-367. doi: 10.1016/j.brainres.2017.01.002. Epub 2017 Jan 4. Brain Res. 2017. PMID: 28063855 Free PMC article. - Vitex Agnus Castus Extract Improves Learning and Memory and Increases the Transcription of Estrogen Receptor α in Hippocampus of Ovariectomized Rats.
Allahtavakoli M, Honari N, Pourabolli I, Kazemi Arababadi M, Ghafarian H, Roohbakhsh A, Esmaeili Nadimi A, Shamsizadeh A. Allahtavakoli M, et al. Basic Clin Neurosci. 2015 Jul;6(3):185-92. Basic Clin Neurosci. 2015. PMID: 26904176 Free PMC article. - Relating sex-bias in human cortical and hippocampal microstructure to sex hormones.
Küchenhoff S, Bayrak Ş, Zsido RG, Saberi A, Bernhardt BC, Weis S, Schaare HL, Sacher J, Eickhoff S, Valk SL. Küchenhoff S, et al. Nat Commun. 2024 Aug 23;15(1):7279. doi: 10.1038/s41467-024-51459-7. Nat Commun. 2024. PMID: 39179555 Free PMC article.
References
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. - PubMed
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG02224/AG/NIA NIH HHS/United States
- R37 AG002224/AG/NIA NIH HHS/United States
- P01 AG17164/AG/NIA NIH HHS/United States
- K12 GM00679/GM/NIGMS NIH HHS/United States
- K12 GM000679/GM/NIGMS NIH HHS/United States
- P01 AG017164/AG/NIA NIH HHS/United States
- R01 AG002224/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous