Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia - PubMed (original) (raw)
Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia
Emanuela Santini et al. J Neurosci. 2007.
Abstract
The molecular basis of L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia (LID), one of the major hindrances in the current therapy for Parkinson's disease, is still unclear. We show that attenuation of cAMP signaling in the medium spiny neurons of the striatum, achieved by genetic inactivation of the dopamine and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32), reduces LID. We also show that, in dyskinetic mice, sensitized cAMP/cAMP-dependent protein kinase/DARPP-32 signaling leads to phosphorylation/activation of the extracellular signal-regulated protein kinases 1 and 2 (ERK1/2). The increase in ERK1/2 phosphorylation associated with dyskinesia results in activation of mitogen- and stress-activated kinase-1 (MSK-1) and phosphorylation of histone H3, two downstream targets of ERK involved in transcriptional regulation. In line with these observations, we found that c-Fos expression is abnormally elevated in the striata of mice affected by LID. Persistent enhancement of the ERK signaling cascade is implicated in the generation of LID. Thus, pharmacological inactivation of ERK1/2 achieved using SL327 (alpha-[amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile), an inhibitor of the mitogen-activated kinase/ERK kinase, MEK, during chronic L-DOPA treatment counteracts the induction dyskinesia. Together, these results indicate that a significant proportion of the abnormal involuntary movements developed in response to chronic L-DOPA are attributable to hyperactivation in striatal medium spiny neurons of a signaling pathway including sequential phosphorylation of DARPP-32, ERK1/2, MSK-1, and histone H3.
Figures
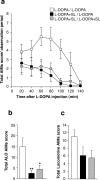
Figure 1.
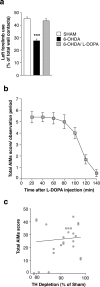
Effects of 6-OHDA lesion and
l
-DOPA administration. a, Left forelimb use was determined using the cylinder test (see Materials and Methods) in sham-lesioned mice (Sham) and in 6-OHDA-lesioned mice before (6-OHDA) or after (6-OHDA/L-DOPA) administration of 20 mg/kg
l
-DOPA plus 12 mg/kg benserazide. Data are means ± SEM (n = 9–36). ***p < 0.001 versus Sham and 6-OHDA/L-DOPA, one-way ANOVA (F(1,83) = 37.48), followed by Bonferroni–Dunn test. b, 6-OHDA-lesioned mice were treated for 10 d with 20 mg/kg
l
-DOPA plus 12 mg/kg benserazide and total axial, limb, orolingual, and locomotive AIMs were scored every 20 min over a period of 140 min after the last drug administration. c, Simple linear regression analysis showing absence of correlation between depletion of TH and AIMs score in 6-OHDA-lesioned mice with a reduction in TH immunoreactivity ≥80% (r = 0.219; p = 0.304).
Figure 2.
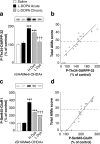
PKA-mediated phosphorylation of DARPP-32 and GluR1 is associated with LID. Sham- or 6-OHDA-lesioned mice were treated with saline, acute
l
-DOPA, or chronic
l
-DOPA. Phospho-Thr34–DARPP-32 (a, b) and phospho-Ser845–GluR1 (c, d) were determined by Western blotting (see Materials and Methods). a, c, 6-OHDA-lesioned mice were divided into low dyskinetic (L Dys) and highly dyskinetic (H Dys) according to their total AIM score (see Materials and Methods) using the median value of 28 as cutoff. Top row shows representative autoradiograms. Bottom row is a summary of data represented as means ± SEM (n = 9–13). ***p < 0.001 versus Sham treated with saline; †††p < 0.001 versus H Dys; one-way ANOVA (a, F(4,52) = 38.44; b, F(4,52) = 69.85), followed by Bonferroni–Dunn test. b, d, Simple regression analysis indicating a significant correlation between AIMs and levels of phospho-Thr34–DARPP-32 (b) (r = 0.921; p < 0.001) and phospho-Ser845–GluR1 (d) (r = 0.953; p < 0.001).
Figure 3.
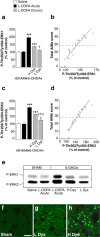
Phosphorylation of ERK1/2 is associated with LID. Sham- or 6-OHDA-lesioned mice were treated with saline, acute
l
-DOPA, or chronic
l
-DOPA. Phospho-Thr202/Tyr204–ERK1 (a, b, e) and ERK2 (c–e) were determined by Western blotting (see Materials and Methods). a, c, 6-OHDA-lesioned mice were divided into low dyskinetic (L Dys) and high dyskinetic (H Dys) according to their total AIM score (see Materials and Methods) using the median value of 28 as cutoff. Data are means ± SEM (n = 9–13). ***p < 0.001 versus Sham treated with saline; †††p < 0.001 and †p < 0.05 versus H Dys; one-way ANOVA (a, F(4,52) = 31.64; c, F(4,52) = 26.96), followed by Bonferroni–Dunn test. b, d, Simple regression analysis indicating a significant correlation between AIMs and levels of phospho-Thr202/Tyr204–ERK1 (b) (r = 0.917; p < 0.001) and phospho-Thr202/Tyr204–ERK2 (d) (r = 0.931; p < 0.001). e, Representative autoradiogram showing phospho-ERK1 (P-ERK1) and phospho-ERK2 (P-ERK2) immunoreactivity. f–h, Immunocytochemical detection of phospho-Thr202/Tyr204–ERK (P-ERK) in the dorsal striata of sham-lesioned mice treated with vehicle (Sham) (f) and of 6-OHDA-lesioned mice treated with
l
-DOPA showing low (L Dys) (g) and high (H Dys) (h) dyskinesia. Scale bar, 40 μm.
Figure 4.
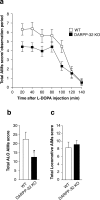
LID is attenuated in DARPP-32 knock-out mice. Wild-type (WT) and DARPP-32 knock-out (DARPP-32 KO) mice received unilateral injections of 6-OHDA (see Materials and Methods) and were treated for 10 d with 20 mg/kg
l
-DOPA combined with 12 mg/kg benserazide. a, Time profile of total axial, limb, orolingual, and locomotive AIMs scored every 20 min over a period of 140 min after the last drug administration. b, Sum of total axial, limb, and orolingual (ALO) AIMs scored during all observation periods. c, Sum of locomotive AIMs scored during all observation periods. Data are expressed as means ± SEM (n = 13–19). a, Repeated-measure ANOVA, significant effect of genotype (F(1,180) = 4.64; p < 0.05), time (F(6,180) = 50.16; p < 0.001), and genotype × time interaction (F(6,180) = 2.47; p < 0.05). b, *p < 0.05 versus WT; Student's t test.
Figure 5.
l
-DOPA-induced phosphorylation of GluR1 and ERK1/2 is reduced in DARPP-32 knock-out mice. Sham- or 6-OHDA-lesioned wild-type (WT) and DARPP-32 knock-out (DARPP-32 KO) mice were treated for 10 d with saline (SHAM/Saline) or
l
-DOPA (20 mg/kg) plus benserazide (12 mg/kg) (6-OHDA/
l
-DOPA) and were killed 30 min after the last injection (see Materials and Methods). Phospho-Ser845–GluR1 (a), phospho-Thr202/Tyr204–ERK1 (b), and phospho-Thr202/Tyr204–ERK2 (c) were determined by Western blotting (see Materials and Methods). Top row shows representative autoradiograms. Bottom row shows summary of data represented as means ± SEM (n = 6–19). **p < 0.01 and ***p < 0.001 versus respective SHAM/Saline group; †††p < 0.001 versus 6-OHDA/L-DOPA WT; two-way ANOVA, followed by Bonferroni–Dunn test. A significant interaction was found between genotype and treatment (a, F(1,41) = 5.24, p < 0.05; b, F(1,41) = 4.54, p < 0.05; and c, F(1,41) = 4.96, p < 0.05).
Figure 6.
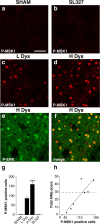
Phosphorylation of MSK-1 is associated with LID. a–d, Immunocytochemical detection of phospho-Thr581–MSK-1 (P-MSK1) in sham-lesioned mouse treated with vehicle (a; SHAM), 6-OHDA-lesioned mouse treated with
l
-DOPA (20 mg/kg) in combination with SL327 (b; SL327) (75 mg/kg), 6-OHDA-lesioned mouse with low dyskinesia (c; L Dys), and 6-OHDA-lesioned mouse with high dyskinesia (d; H Dys). Note the abolishment of phospho-MSK-1 immunoreactivity in b compared with c and d. e, Phospho-Thr202/Tyr204–ERK (P-ERK) immunoreactivity in the dorsal striatum of a mouse with high dyskinesia. f, Double immunolabeling showing colocalization between phospho-ERK and phospho-MSK-1 in a mouse with high dyskinesia. g, 6-OHDA-lesioned mice were divided into low dyskinetic (L Dys) and high dyskinetic (H Dys) according to their total AIM score (see Materials and Methods) using the median value of 29 as cutoff. Histograms show the number of phospho-MSK-1-positive cells in the dorsal striata of sham-lesioned mice (SHAM), mice with low (L Dys) and high (H Dys) dyskinesia, and 6-OHDA-lesioned mice treated with
l
-DOPA in combination with SL327 (75 mg/kg) (SL327). Data are means ± SEM (n = 3–7). ***p < 0.001 versus L Dys; one-way ANOVA, followed by Bonferroni–Dunn test. h, Simple regression analysis showing significant correlation (r = 0.858; p < 0.05) between the number of phospho-MSK-1-positive cells and AIM score. Scale bar: a–f, 40 μm.
Figure 7.
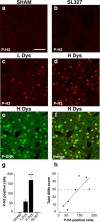
Phosphorylation of histone H3 is associated with LID. a–d, Immunocytochemical detection of phospho-Ser10–histone H3 (P-H3) in sham-lesioned mouse treated with vehicle (a; Sham), 6-OHDA-lesioned mouse treated with
l
-DOPA (20 mg/kg) in combination with SL327 (75 mg/kg) (b; SL327), 6-OHDA-lesioned mouse with low dyskinesia (c; L Dys), and 6-OHDA-lesioned mouse with high dyskinesia (d; H Dys). Note the abolishment of phospho-Ser10–histone H3 immunoreactivity in b compared with c and d. e, Phospho-Thr202/Tyr204–ERK (P-ERK) immunoreactivity in the dorsal striatum of a mouse with high dyskinesia. f, Double immunolabeling showing colocalization between Phospho-Thr202/Tyr204–ERK and phospho-Ser10–histone H3 in a mouse with high dyskinesia. g, 6-OHDA-lesioned mice were divided into low dyskinetic (L Dys) and high dyskinetic (H Dys) according to their total AIM score (see Materials and Methods) using the median value of 29 as cutoff. Histograms show the number of phospho-Ser10–histone H3-positive cells in the dorsal striata of sham-lesioned mice (SHAM), mice with low (L Dys) and high (H Dys) dyskinesia, and 6-OHDA-lesioned mice treated with
l
-DOPA in combination with SL327 (75 mg/kg) (SL327). Data are means ± SEM (n = 3–7). ***p < 0.001 versus L Dys; one-way ANOVA, followed by Bonferroni–Dunn test. h, Simple regression analysis showing significant correlation (r = 0.803; p < 0.05) between the number of phospho-Ser10–histone H3-positive cells and AIM score. Scale bar: a–f, 40 μm.
Figure 8.
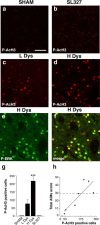
Phosphorylation of acetyl-histone H3 is associated with LID. a–d, Immunocytochemical detection of phospho-Ser10–acetylLys14 histone H3 (P-AcH3) in sham-lesioned mouse treated with vehicle (a; SHAM), 6-OHDA-lesioned mouse treated with
l
-DOPA (20 mg/kg) in combination with SL327 (75 mg/kg) (b; SL327), 6-OHDA-lesioned mouse with low dyskinesia (c; L Dys), and 6-OHDA-lesioned mouse with high dyskinesia (d; H Dys). Note the abolishment of phospho-Ser10–acetylLys14 histone H3 immunoreactivity in b compared with c and d. e, Phospho-Thr202/Tyr204–ERK (P-ERK) immunoreactivity in the dorsal striatum of a mouse with high dyskinesia. f, Double immunolabeling showing colocalization between Phospho-Thr202/Tyr204–ERK and phospho-Ser10–acetylLys14 histone H3 in a mouse with H Dys. g, 6-OHDA-lesioned mice were divided into low dyskinetic (L Dys) and high dyskinetic (H Dys) according to their total AIMs score (see Materials and Methods) using the median value of 29 as cutoff. Histograms show the number of phospho-Ser10–acetylLys14 histone H3-positive cells in the dorsal striata of sham-lesioned mice (SHAM), mice with low (L Dys) and high (H Dys) dyskinesia, and 6-OHDA-lesioned mice treated with
l
-DOPA in combination with SL327 (75 mg/kg) (SL327). Data are means ± SEM (n = 3–7). ***p < 0.001 versus L Dys; one-way ANOVA, followed by Bonferroni–Dunn test. h, Simple regression analysis showing significant correlation (r = 0.835; p < 0.05) between the number of phospho-Ser10–acetylLys14 histone H3-positive cells and AIM score. Scale bar: a–f, 40 μm.
Figure 9.
Pretreatment with SL327 reduces LID. 6-OHDA-lesioned mice were treated for 9 d with 20 mg/kg
l
-DOPA alone (white symbols) or in combination with SL327 (75 mg/kg) (SL; black and gray symbols). At the end of this period, AIMs were determined after administration of
l
-DOPA alone (open and black symbols) or
l
-DOPA plus SL327 (gray symbols). a, Time profile of total axial, limb, orolingual, and locomotive AIMs scored every 20 min over a period of 140 min after the last drug administration. b, Sum of total axial, limb, and orolingual (ALO) AIMs scored during all observation periods. c, Sum of locomotive AIMs scored during all observation periods. Data are expressed as means ± SEM (n = 8). a, Repeated-measure ANOVA, significant effect of SL327 treatment (F(2,126) = 4.59; p < 0.05), time (F(6,126) = 14.95; p < 0.001), and SL327 treatment × time interaction (F(12,126) = 2.23; p < 0.05). b, *p < 0.05 and **p < 0.01 versus L-DOPA/L-DOPA group; one-way ANOVA, followed by Bonferroni–Dunn test.
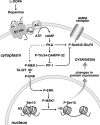
Figure 10.
Schematic diagram illustrating the involvement of the cAMP/DARPP-32 and ERK pathways in LID. In Parkinson's disease, depletion of dopamine results in increased responsiveness of dopamine D1 receptors, which are expressed by a large proportion of striatal medium spiny neurons. Administration of
l
-DOPA leads to D1 receptor-mediated activation of PKA, which phosphorylates the GluR1 subunit of AMPA receptors at Ser845 and DARPP-32 at Thr34. PKA-mediated phosphorylation of GluR1 promotes glutamatergic transmission and is intensified by phospho-Thr34–DARPP-32 via inhibition of PP-1. In addition, increased PKA/DARPP-32 signaling leads to activation of MEK, which is facilitated by inhibition of PP-1 and is likely to depend on other signals induced by cAMP and Ca2+ (not shown). Phospho-ERK translocation to the nucleus results in sequential phosphorylation/activation of MSK-1 and histone H3. This, in turn, leads to chromatin rearrangements and transcription of immediate early genes, such as c-fos (supplemental Fig. 2, available at
as supplemental material). Persistent abnormal activation of these pathways by
l
-DOPA appears to be involved in long-term changes responsible for dyskinesia. Thus, LID is attenuated by genetic inactivation of DARPP-32 or by blockade of ERK signaling, achieved with SL327.
Similar articles
- Gα(olf) mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4-dihydroxyphenylalanine-induced dyskinesia.
Alcacer C, Santini E, Valjent E, Gaven F, Girault JA, Hervé D. Alcacer C, et al. J Neurosci. 2012 Apr 25;32(17):5900-10. doi: 10.1523/JNEUROSCI.0837-12.2012. J Neurosci. 2012. PMID: 22539851 Free PMC article. - A Role for Mitogen- and Stress-Activated Kinase 1 in L-DOPA-Induced Dyskinesia and ∆FosB Expression.
Feyder M, Södersten E, Santini E, Vialou V, LaPlant Q, Watts EL, Spigolon G, Hansen K, Caboche J, Nestler EJ, Fisone G. Feyder M, et al. Biol Psychiatry. 2016 Mar 1;79(5):362-371. doi: 10.1016/j.biopsych.2014.07.019. Epub 2014 Jul 28. Biol Psychiatry. 2016. PMID: 25193242 Free PMC article. - Dopamine- and cAMP-regulated phosphoprotein of 32-kDa (DARPP-32)-dependent activation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1) signaling in experimental parkinsonism.
Santini E, Feyder M, Gangarossa G, Bateup HS, Greengard P, Fisone G. Santini E, et al. J Biol Chem. 2012 Aug 10;287(33):27806-12. doi: 10.1074/jbc.M112.388413. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22753408 Free PMC article. - DARPP-32 and modulation of cAMP signaling: involvement in motor control and levodopa-induced dyskinesia.
Håkansson K, Lindskog M, Pozzi L, Usiello A, Fisone G. Håkansson K, et al. Parkinsonism Relat Disord. 2004 Jul;10(5):281-6. doi: 10.1016/j.parkreldis.2004.02.010. Parkinsonism Relat Disord. 2004. PMID: 15196506 Review. - Signal transduction in L-DOPA-induced dyskinesia: from receptor sensitization to abnormal gene expression.
Spigolon G, Fisone G. Spigolon G, et al. J Neural Transm (Vienna). 2018 Aug;125(8):1171-1186. doi: 10.1007/s00702-018-1847-7. Epub 2018 Feb 2. J Neural Transm (Vienna). 2018. PMID: 29396608 Free PMC article. Review.
Cited by
- Are cyclooxygenase-2 and nitric oxide involved in the dyskinesia of Parkinson's disease induced by L-DOPA?
Bortolanza M, Padovan-Neto FE, Cavalcanti-Kiwiatkoski R, Dos Santos-Pereira M, Mitkovski M, Raisman-Vozari R, Del-Bel E. Bortolanza M, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Jul 5;370(1672):20140190. doi: 10.1098/rstb.2014.0190. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26009769 Free PMC article. - Gene expression analyses identify Narp contribution in the development of L-DOPA-induced dyskinesia.
Charbonnier-Beaupel F, Malerbi M, Alcacer C, Tahiri K, Carpentier W, Wang C, During M, Xu D, Worley PF, Girault JA, Hervé D, Corvol JC. Charbonnier-Beaupel F, et al. J Neurosci. 2015 Jan 7;35(1):96-111. doi: 10.1523/JNEUROSCI.5231-13.2015. J Neurosci. 2015. PMID: 25568106 Free PMC article. - Interrogating the aged striatum: robust survival of grafted dopamine neurons in aging rats produces inferior behavioral recovery and evidence of impaired integration.
Collier TJ, O'Malley J, Rademacher DJ, Stancati JA, Sisson KA, Sortwell CE, Paumier KL, Gebremedhin KG, Steece-Collier K. Collier TJ, et al. Neurobiol Dis. 2015 May;77:191-203. doi: 10.1016/j.nbd.2015.03.005. Epub 2015 Mar 11. Neurobiol Dis. 2015. PMID: 25771169 Free PMC article. - Gα(olf) mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4-dihydroxyphenylalanine-induced dyskinesia.
Alcacer C, Santini E, Valjent E, Gaven F, Girault JA, Hervé D. Alcacer C, et al. J Neurosci. 2012 Apr 25;32(17):5900-10. doi: 10.1523/JNEUROSCI.0837-12.2012. J Neurosci. 2012. PMID: 22539851 Free PMC article. - L-DOPA Oppositely Regulates Synaptic Strength and Spine Morphology in D1 and D2 Striatal Projection Neurons in Dyskinesia.
Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R. Suarez LM, et al. Cereb Cortex. 2016 Oct 17;26(11):4253-4264. doi: 10.1093/cercor/bhw263. Cereb Cortex. 2016. PMID: 27613437 Free PMC article.
References
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6:461–474. - PubMed
- Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. - PubMed
- Brotchie JM, Lee J, Venderova K. Levodopa-induced dyskinesia in Parkinson's disease. J Neural Transm. 2005;112:359–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous