An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system - PubMed (original) (raw)
An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system
Tobias H Kloepper et al. Mol Biol Cell. 2007 Sep.
Abstract
Proteins of the SNARE (soluble N-ethylmalemide-sensitive factor attachment protein receptor) family are essential for the fusion of transport vesicles with an acceptor membrane. Despite considerable sequence divergence, their mechanism of action is conserved: heterologous sets assemble into membrane-bridging SNARE complexes, in effect driving membrane fusion. Within the cell, distinct functional SNARE units are involved in different trafficking steps. These functional units are conserved across species and probably reflect the conservation of the particular transport step. Here, we have systematically analyzed SNARE sequences from 145 different species and have established a highly accurate classification for all SNARE proteins. Principally, all SNAREs split into four basic types, reflecting their position in the four-helix bundle complex. Among these four basic types, we established 20 SNARE subclasses that probably represent the original repertoire of a eukaryotic cenancestor. This repertoire has been modulated independently in different lines of organisms. Our data are in line with the notion that the ur-eukaryotic cell was already equipped with the various compartments found in contemporary cells. Possibly, the development of these compartments is closely intertwined with episodes of duplication and divergence of a prototypic SNARE unit.
Figures
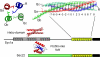
Figure 1.
Schematic representation of the different domain arrangements found in SNARE proteins. The four-helix bundle structure of the neuronal SNARE complex is shown as ribbon diagram on the top right (blue, red, and green for synaptobrevin 2, syntaxin 1a, and SNAP-25a, respectively). The layers (−7 until + 8) in the core of the bundle are indicated by virtual bonds between the corresponding Cα positions. The structure of the central 0-layer is shown in detail on the top left (Sutton et al., 1998). Below the domain architecture of two most important types of SNARE proteins are depicted. The highly conserved SNARE motif is indicated by a yellow box and the adjacent transmembrane domain by a black box. In addition, the structures of two types of autonomous N-terminal domains: a three-helix bundle (Fernandez et al., 1998; Lerman et al., 2000) and a profilin-like domain (Gonzalez et al., 2001) are shown.
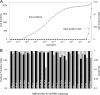
Figure 2.
Quality assessment of the generated SNARE protein classification. (A) The absolute number of true positives is shown in dependence to the expectation value of the correct HMM profile of a search through the Feb. 2006 nr-database together with the rate of false positives found by an eye-by-eye verification of the results. The classification separates the largest part of the true positively found sequence motifs very well from the false positives. False positives are only found with rather large expectation values. The first false positive is found at an expectation value of 0.012. The false positive rate reaches the 1% margin at an expectation value of ∼0.1 and the 5% margin at ∼5. (B) To estimate the PPR (■), the rate at which a positive is a true positive, and the sensitivity (▩), the percentage of correctly found true positives, of the generated HMM profiles one need to know the true and false negatives of a search. Because this is almost impossible to know for a random database, we used a resampling method. We randomly gathered 95% of the SNARE motifs in each class to generate a new HMM profile. The 95% sampling assured each class to consist of at least 10 sequences and was also efficient to minimize variation within the generated motif. The other SNAREs were used as the search database. For a comparable expectation value we set the database size to a fixed size of one million sequences. We repeated the resampling 1000 times to minimize variation. We assumed the profile with the best expectation value to be the correct class. All 20 profiles achieved at least a 95% PPR and, besides one, a sensitivity of 97%. If we count a true positive as a true positive whenever it has at least achieved the second best expectation value, the PPR rises above the 99% margin for all profiles.
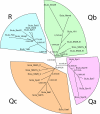
Figure 3.
General outline of the phylogenetic tree of SNARE proteins. To gain insights into the evolutionary relationship of SNARE proteins, we constructed trees of the SNARE sets of 11 representative species and one larger tree containing the SNAREs of all 11 species. The IQPNNI-trees were generated as described in Materials and Methods. All original IQPNNI-trees can be downloaded from our webpage (
http://bioinformatics.mpibpc.mpg.de/snare/
). As an example, the tree of the 25 SNAREs of Schistosoma japonicum (ScJa) is shown. It shows the typical layout that we found for the species examined in our analysis. Basically, all SNAREs split into four well-supported groups that represent the four different positions of the four-helix bundle SNARE core complexes (see Figure 1). Each of the four main groups segregates into the distinct subgroups (see Table 1 for nomenclature) found by our classification. The labels on the tree edges represent the likelihood mapping (first) and AU support values (right). Interestingly the inner most split (Qa and Qc vs. R and Qc) is well supported. This clustering can be observed in all species trees calculated, except those of the two fungi species. Furthermore, the SNARE set involved in ER-transport, group (I), is quite diverged from the other SNARE groups and seems to have undergone a rather specific evolution. Note that the two SNARE motifs of Qbc-SNAREs are indicated by a B or C for the Qb- or the Qc-helix, respectively.
Similar articles
- SNAREing the basis of multicellularity: consequences of protein family expansion during evolution.
Kloepper TH, Kienle CN, Fasshauer D. Kloepper TH, et al. Mol Biol Evol. 2008 Sep;25(9):2055-68. doi: 10.1093/molbev/msn151. Epub 2008 Jul 10. Mol Biol Evol. 2008. PMID: 18621745 - An evolutionary perspective on eukaryotic membrane trafficking.
Gurkan C, Koulov AV, Balch WE. Gurkan C, et al. Adv Exp Med Biol. 2007;607:73-83. doi: 10.1007/978-0-387-74021-8_6. Adv Exp Med Biol. 2007. PMID: 17977460 Review. - Phylogeny of the SNARE vesicle fusion machinery yields insights into the conservation of the secretory pathway in fungi.
Kienle N, Kloepper TH, Fasshauer D. Kienle N, et al. BMC Evol Biol. 2009 Jan 23;9:19. doi: 10.1186/1471-2148-9-19. BMC Evol Biol. 2009. PMID: 19166604 Free PMC article. - A look beyond the QR code of SNARE proteins.
Yadav D, Hacisuleyman A, Dergai M, Khalifeh D, Abriata LA, Peraro MD, Fasshauer D. Yadav D, et al. Protein Sci. 2024 Sep;33(9):e5158. doi: 10.1002/pro.5158. Protein Sci. 2024. PMID: 39180485 Free PMC article. - Differences in the SNARE evolution of fungi and metazoa.
Kienle N, Kloepper TH, Fasshauer D. Kienle N, et al. Biochem Soc Trans. 2009 Aug;37(Pt 4):787-91. doi: 10.1042/BST0370787. Biochem Soc Trans. 2009. PMID: 19614595 Review.
Cited by
- Secretory protein biogenesis and traffic in the early secretory pathway.
Barlowe CK, Miller EA. Barlowe CK, et al. Genetics. 2013 Feb;193(2):383-410. doi: 10.1534/genetics.112.142810. Genetics. 2013. PMID: 23396477 Free PMC article. Review. - Examining the Underappreciated Role of _S_-Acylated Proteins as Critical Regulators of Phagocytosis and Phagosome Maturation in Macrophages.
Dixon CL, Mekhail K, Fairn GD. Dixon CL, et al. Front Immunol. 2021 Apr 1;12:659533. doi: 10.3389/fimmu.2021.659533. eCollection 2021. Front Immunol. 2021. PMID: 33868308 Free PMC article. Review. - Molecular structures and function of the autophagosome-lysosome fusion machinery.
Diao J, Yip CK, Zhong Q. Diao J, et al. Autophagy Rep. 2024;3(1):2305594. doi: 10.1080/27694127.2024.2305594. Epub 2024 Feb 4. Autophagy Rep. 2024. PMID: 38344192 - Evolution: like any other science it is predictable.
Morris SC. Morris SC. Philos Trans R Soc Lond B Biol Sci. 2010 Jan 12;365(1537):133-45. doi: 10.1098/rstb.2009.0154. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20008391 Free PMC article. - Fluorescence Lifetime and Cross-correlation Spectroscopy for Observing Membrane Fusion of Liposome Models Containing Synaptic Proteins.
Grothe T, Walla PJ. Grothe T, et al. Methods Mol Biol. 2022;2417:167-180. doi: 10.1007/978-1-0716-1916-2_13. Methods Mol Biol. 2022. PMID: 35099799
References
- Antonin W., Dulubova I., Arac D., Pabst S., Plitzner J., Rizo J., Jahn R. The N-terminal domains of syntaxin 7 and vti1b form three-helix bundles that differ in their ability to regulate SNARE complex assembly. J. Biol. Chem. 2002a;277:36449–36456. - PubMed
- Antonin W., Fasshauer D., Becker S., Jahn R., Schneider T. R. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat. Struct. Biol. 2002b;9:107–111. - PubMed
- Aury J. M., et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature. 2006;444:171–178. - PubMed
- Ayong L., Pagnotti G., Tobon A. B., Chakrabarti D. Identification of Plasmodium falciparum family of SNAREs. Mol. Biochem. Parasitol. 2007;152:113–122. - PubMed
- Banfield D. K. SNARE complexes—is there sufficient complexity for vesicle targeting specificity? Trends Biochem. Sci. 2001;26:67–68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases