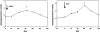Neurosteroids in the context of stress: implications for depressive disorders - PubMed (original) (raw)
Review
Neurosteroids in the context of stress: implications for depressive disorders
Susan S Girdler et al. Pharmacol Ther. 2007 Oct.
Abstract
Animal models indicate that the neuroactive steroids 3alpha,5alpha-THP (allopregnanolone) and 3alpha,5alpha-THDOC (allotetrahydroDOC) are stress responsive, serving as homeostatic mechanisms in restoring normal GABAergic and hypothalamic-pituitary-adrenal (HPA) function following stress. While neurosteroid increases to stress are adaptive in the short term, animal models of chronic stress and depression find lower brain and plasma neurosteroid concentrations and alterations in neurosteroid responses to acute stressors. It has been suggested that disruption in this homeostatic mechanism may play a pathogenic role in some psychiatric disorders related to stress. In humans, neurosteroid depletion is consistently documented in patients with current depression and may reflect their greater chronic stress. Women with the depressive disorder, premenstrual dysphoric disorder (PMDD), have greater daily stress and a greater rate of traumatic stress. While results on baseline concentrations of neuroactive steroids in PMDD are mixed, PMDD women have diminished functional sensitivity of GABA(A) receptors and our laboratory has found blunted allopregnanolone responses to mental stress relative to non-PMDD controls. Similarly, euthymic women with histories of clinical depression, which may represent a large proportion of PMDD women, show more severe dysphoric mood symptoms and blunted allopregnanolone responses to stress versus never-depressed women. It is suggested that failure to mount an appropriate allopregnanolone response to stress may reflect the price of repeated biological adaptations to the increased life stress that is well documented in depressive disorders and altered allopregnanolone stress responsivity may also contribute to the dysregulation seen in HPA axis function in depression.
Figures
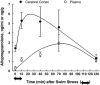
Fig. 1
Allopregnanolone levels in plasma and brain of adult Sprague-Dawley male rats after acute swim stress. Rats were subjected to acute stress by swimming for 10 min in ambient temperature water. Allopregnanolone levels were measured by RIA after purification of the steroid by HPLC. The data represent the mean±SEM for 3 separate experiments (_n_=12 rats per time point) for the cerebral cortex and plasma. Stress-induced increases of allopregnanolone in the cerebral cortex and plasma were statistically significant at 10, 40, and 70 min after the initiation of swim stress compared with nonstressed levels at 0 time [analysis of variance (ANOVA), P<0.05; Tukey's honestly significant difference procedure (HSD), P<0.05]. Adapted from Purdy et al. (1991).
Fig. 2
Mean±SEM allopregnanolone levels in response to the GnRH test (*, P<0.01 vs. 0 min) and in response to the CRF test (*, P<0.01 vs. 0 min). Adapted from Genazzani et al. (1998).
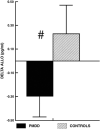
Fig. 3
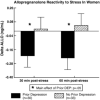
Mean (± SEM) change in luteal phase plasma allopregnanolone (ALLO) during stress in PMDD women and control subjects (#, P<0.010). From Girdler et al. (2001).
Fig. 4
Allopregnanolone response to the adrenocorticotropic hormone (ACTH) test in patients with premenstrual syndrome (shaded bars) and in the control group (white bars) expressed as area under the curve (*, P<0.05). Adapted from Lombardi et al. (2004).
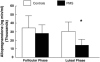
Fig. 5
Change (stress−baseline) in allopregnanolone (ALLO) concentrations at 30 and 60 min post-stress in women as a function of histories of depression (DEP). From Klatzkin et al. (2006a).
Similar articles
- GABAergic neuroactive steroid response to sertraline in premenstrual dysphoric disorder.
Miller KN, Standeven L, Morrow AL, Payne JL, Epperson CN, Hantsoo L. Miller KN, et al. Psychoneuroendocrinology. 2024 Feb;160:106684. doi: 10.1016/j.psyneuen.2023.106684. Epub 2023 Nov 30. Psychoneuroendocrinology. 2024. PMID: 38091917 Free PMC article. - Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder.
Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Girdler SS, et al. Biol Psychiatry. 2001 May 1;49(9):788-97. doi: 10.1016/s0006-3223(00)01044-1. Biol Psychiatry. 2001. PMID: 11331087 - The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD.
Almeida FB, Pinna G, Barros HMT. Almeida FB, et al. Int J Mol Sci. 2021 May 23;22(11):5495. doi: 10.3390/ijms22115495. Int J Mol Sci. 2021. PMID: 34071053 Free PMC article. Review. - Effects of GABA active steroids in the female brain with a focus on the premenstrual dysphoric disorder.
Bixo M, Johansson M, Timby E, Michalski L, Bäckström T. Bixo M, et al. J Neuroendocrinol. 2018 Feb;30(2). doi: 10.1111/jne.12553. J Neuroendocrinol. 2018. PMID: 29072794 Review. - Luteal phase sertraline treatment of premenstrual dysphoric disorder (PMDD): Effects on markers of hypothalamic pituitary adrenal (HPA) axis activation and inflammation.
Barone JC, Ho A, Osborne LM, Eisenlohr-Moul TA, Morrow AL, Payne JL, Epperson CN, Hantsoo L. Barone JC, et al. Psychoneuroendocrinology. 2024 Nov;169:107145. doi: 10.1016/j.psyneuen.2024.107145. Epub 2024 Jul 24. Psychoneuroendocrinology. 2024. PMID: 39096755
Cited by
- Neurosteroids, stress and depression: potential therapeutic opportunities.
Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Zorumski CF, et al. Neurosci Biobehav Rev. 2013 Jan;37(1):109-22. doi: 10.1016/j.neubiorev.2012.10.005. Epub 2012 Oct 17. Neurosci Biobehav Rev. 2013. PMID: 23085210 Free PMC article. Review. - Effects of acute progesterone administration upon responses to acute psychosocial stress in men.
Childs E, Van Dam NT, de Wit H. Childs E, et al. Exp Clin Psychopharmacol. 2010 Feb;18(1):78-86. doi: 10.1037/a0018060. Exp Clin Psychopharmacol. 2010. PMID: 20158297 Free PMC article. - Allopregnanolone's attenuation of the lordosis-inhibiting effects of restraint is blocked by the antiprogestin, CDB-4124.
Uphouse L, Hiegel C. Uphouse L, et al. Pharmacol Biochem Behav. 2014 Jul;122:16-9. doi: 10.1016/j.pbb.2014.03.012. Epub 2014 Mar 18. Pharmacol Biochem Behav. 2014. PMID: 24650591 Free PMC article. - Early postnatal allopregnanolone levels alteration and adult behavioral disruption in rats: Implication for drug abuse.
Bartolomé I, Llidó A, Darbra S, Pallarès M. Bartolomé I, et al. Neurobiol Stress. 2019 Dec 27;12:100208. doi: 10.1016/j.ynstr.2019.100208. eCollection 2020 May. Neurobiol Stress. 2019. PMID: 32435661 Free PMC article. - Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats.
Santoru F, Berretti R, Locci A, Porcu P, Concas A. Santoru F, et al. Psychopharmacology (Berl). 2014 Sep;231(17):3351-64. doi: 10.1007/s00213-014-3539-9. Epub 2014 Apr 13. Psychopharmacology (Berl). 2014. PMID: 24728651
References
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdale mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. - PubMed
- Andreen L, Sundstrom-Poromaa I, Bixo M, Nyberg S, Backstrom T. Allopregnanolone concentration and mood-a bimodal association in postmenopausal women treated with oral progesterone. Psychopharmacology. 2006;187:209–221. - PubMed
- Bancroft J, Cook A, Davidson D, Bennie J, Goodwin G. Blunting of neuroendocrine responses to infusion of L-tryptophan in women with perimenstrual mood change. Psychol Med. 1991;21:305–312. - PubMed
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, et al. Time-dependent changes in rat brain neuroactive steroid concentrations and GABA A receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical