Predicting absolute ligand binding free energies to a simple model site - PubMed (original) (raw)
Predicting absolute ligand binding free energies to a simple model site
David L Mobley et al. J Mol Biol. 2007.
Abstract
A central challenge in structure-based ligand design is the accurate prediction of binding free energies. Here we apply alchemical free energy calculations in explicit solvent to predict ligand binding in a model cavity in T4 lysozyme. Even in this simple site, there are challenges. We made systematic improvements, beginning with single poses from docking, then including multiple poses, additional protein conformational changes, and using an improved charge model. Computed absolute binding free energies had an RMS error of 1.9 kcal/mol relative to previously determined experimental values. In blind prospective tests, the methods correctly discriminated between several true ligands and decoys in a set of putative binders identified by docking. In these prospective tests, the RMS error in predicted binding free energies relative to those subsequently determined experimentally was only 0.6 kcal/mol. X-ray crystal structures of the new ligands bound in the cavity corresponded closely to predictions from the free energy calculations, but sometimes differed from those predicted by docking. Finally, we examined the impact of holding the protein rigid, as in docking, with a view to learning how approximations made in docking affect accuracy and how they may be improved.
Figures
Fig. 1. The model hydrophobic binding site in the L99A mutant of T4 lysozyme
The enclosed molecular surface of the cavity is shown (brown) as is the crystallographic geometry of a bound benzene ligand (green), within the context of the overall structure of T4 lysozyme (green ribbons, PDB code 181L). The sidechain of Met102 is also shown for reference.
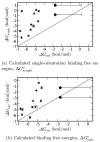
Fig. 2. Calculated binding free energies compared with experiment
Calculated and experimental binding free energies are shown with error bars; the calculated error bars represent one standard deviation. The two points shown as larger diamonds are the non-ligands phenol and 2-fluorobenzaldehyde; for these, only a lower limit on the experimental binding free energy is known, as denoted by a large experimental error bar to the right. The diagonal x = y line denotes perfect agreement with experiment. (a) Calculated ΔGsingleo, single-orientation binding free energies, including only the contribution from the single best docking orientation. (b) ΔGcalc.o, binding free energies, including all relevant ligand orientations, and contributions from releasing Val111 from its kinetic confinement.
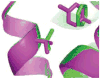
Fig. 3. Val111 reorients on ligand binding
Val111 is observed to adopt a different sidechain rotamer from the apo crystallographic structure in co-crystal structures with several different ligands. Shown here is the benzene-bound structure (PDB code 181L), green, which is virtually identical to the apo structure of the protein. Also shown is the _p_-xylene bound structure (PDB code 187L) in magenta. The sticks at left show the reorientation of the Val111 sidechain on binding to _p_-xylene by roughly 120° relative to the benzene-bound and apo structures.
Fig. 4. DOCK scores for the best-ranked pose for each molecule versus experimental binding free energies
The correlation coefficient (R) is −0.69, meaning that compounds that DOCK predicts should bind strongly tend to bind weakly. Additionally, the two nonbinders have similar DOCK scores to a number of the binders.
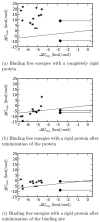
Fig. 5. Comparison of calculated and experimental binding free energies with the protein held rigid
(a) Binding free energies with the protein completely rigid. The RMS error relative to experiment is 19.78±0.06 kcal/mol and the correlation coefficient (R) is −0.05±0.09. (b) Binding free energies with the whole protein minimized separately for each ligand. The RMS error relative to experiment is 4.92±0.07 kcal/mol and the correlation coefficient (R) is 0.82±0.09. (c) Binding free energies with only the binding site minimized for each ligand. The RMS error relative to experiment is 4.06±0.06 kcal/mol and the correlation coefficient (R) is 0.32±0.08. The x = y indicates perfect agreement with experiment.
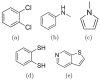
Fig. 6. Five compounds for which binding predictions were made
(a) 1,2-dichlorobenzene; (b) n-methylaniline; (c) 1-methylpyrrole; (d) 1,2-benzenedithiol; and (e) thieno[2,3-c]pyridine.
Fig. 7. Predicted and experimental ligand orientations
Stereo images comparing the experimental and predicted poses for three ligands bound to L99A. (a) The two observed configurations of 1,2-dichlorobenzene, structure determined to 1.70 Å resolution. (b) 1-methylpyrrole, structure determined to 1.94 Å A resolution and (c) n-methylaniline, structure determined to 2.07 Å resolution. The crystallographic carbon atoms of protein residue M102 and each ligand are colored grey. The carbon atoms of the docking predictions are colored yellow, and the carbon atoms of the free energy predictions are colored magenta. The carbon atoms of the second free energy prediction in (c) are colored cyan. The Fo_−_Fc density maps are contoured at 3_σ_ (green mesh). PDB codes are 2OTY, 2OU0, and 2OTZ, respectively.
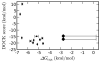
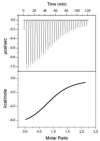
Fig. 8. Representative ITC data
Data and fit for T4 lysozyme L99A (0.063 mM) titrated with 1,2-dichlorobenzene (~ 0.6 mM). An initial injection of 2.5_μ_L was followed by 29 injections of 10_μ_L of the ligand solution made every 2.5 min into the 1.4 mL reaction cell. After subtraction of blank runs, titrations were fit as described under Experimental Procedures to obtain the results in Table 4
Similar articles
- Predicting ligand binding affinity with alchemical free energy methods in a polar model binding site.
Boyce SE, Mobley DL, Rocklin GJ, Graves AP, Dill KA, Shoichet BK. Boyce SE, et al. J Mol Biol. 2009 Dec 11;394(4):747-63. doi: 10.1016/j.jmb.2009.09.049. Epub 2009 Sep 24. J Mol Biol. 2009. PMID: 19782087 Free PMC article. - Blind prediction of charged ligand binding affinities in a model binding site.
Rocklin GJ, Boyce SE, Fischer M, Fish I, Mobley DL, Shoichet BK, Dill KA. Rocklin GJ, et al. J Mol Biol. 2013 Nov 15;425(22):4569-83. doi: 10.1016/j.jmb.2013.07.030. Epub 2013 Jul 26. J Mol Biol. 2013. PMID: 23896298 Free PMC article. - How to deal with multiple binding poses in alchemical relative protein-ligand binding free energy calculations.
Kaus JW, Harder E, Lin T, Abel R, McCammon JA, Wang L. Kaus JW, et al. J Chem Theory Comput. 2015 Jun 9;11(6):2670-9. doi: 10.1021/acs.jctc.5b00214. J Chem Theory Comput. 2015. PMID: 26085821 Free PMC article. - Conformational energy range of ligands in protein crystal structures: The difficult quest for accurate understanding.
Peach ML, Cachau RE, Nicklaus MC. Peach ML, et al. J Mol Recognit. 2017 Aug;30(8):10.1002/jmr.2618. doi: 10.1002/jmr.2618. Epub 2017 Feb 24. J Mol Recognit. 2017. PMID: 28233410 Free PMC article. Review. - Advances in Docking.
Sulimov VB, Kutov DC, Sulimov AV. Sulimov VB, et al. Curr Med Chem. 2019;26(42):7555-7580. doi: 10.2174/0929867325666180904115000. Curr Med Chem. 2019. PMID: 30182836 Review.
Cited by
- Accurate prediction of protein-ligand interactions by combining physical energy functions and graph-neural networks.
Hong Y, Ha J, Sim J, Lim CJ, Oh KS, Chandrasekaran R, Kim B, Choi J, Ko J, Shin WH, Lee J. Hong Y, et al. J Cheminform. 2024 Nov 4;16(1):121. doi: 10.1186/s13321-024-00912-2. J Cheminform. 2024. PMID: 39497201 Free PMC article. - Absolute Binding Free Energy Calculations for Highly Flexible Protein MDM2 and Its Inhibitors.
Singh N, Li W. Singh N, et al. Int J Mol Sci. 2020 Jul 4;21(13):4765. doi: 10.3390/ijms21134765. Int J Mol Sci. 2020. PMID: 32635537 Free PMC article. - Analysis of Glycan Recognition by Concanavalin A Using Absolute Binding Free Energy Calculations.
Musleh S, Alibay I, Biggin PC, Bryce RA. Musleh S, et al. J Chem Inf Model. 2024 Oct 28;64(20):8063-8073. doi: 10.1021/acs.jcim.4c01088. Epub 2024 Oct 16. J Chem Inf Model. 2024. PMID: 39413277 Free PMC article. - Docking-undocking combination applied to the D3R Grand Challenge 2015.
Ruiz-Carmona S, Barril X. Ruiz-Carmona S, et al. J Comput Aided Mol Des. 2016 Sep;30(9):805-815. doi: 10.1007/s10822-016-9979-z. Epub 2016 Oct 5. J Comput Aided Mol Des. 2016. PMID: 27709317 - Accelerated Enveloping Distribution Sampling (AEDS) Allows for Efficient Sampling of Orthogonal Degrees of Freedom.
Gracia Carmona O, Oostenbrink C. Gracia Carmona O, et al. J Chem Inf Model. 2023 Jan 9;63(1):197-207. doi: 10.1021/acs.jcim.2c01272. Epub 2022 Dec 13. J Chem Inf Model. 2023. PMID: 36512416 Free PMC article.
References
- Halperin I, Ma B, Wolfson H, Nussinov R. Principles of docking: An overview of search algorithms and a guide to scoring functions. Proteins: Str, Funct, and Genet. 2002;47:409–443. - PubMed
- Shoichet BK, Leach AR, Kuntz ID. Ligand solvation in molecular docking. Proteins: Struct, Funct, and Genet. 1999;34:4–16. - PubMed
- Huang N, Kalyanaraman C, Bernacki K, Jacobson MP. Molecular mechanics methods for predicting protein-ligand binding. Phys Chem Chem Phys. 2006;8:5166–5177. - PubMed
- Srinivasan J, Cheatam TE, III, Cieplak P, Kollman PA, Case DA. Continuum solvent studies of the stability of DNA, RNA, and Phosphoramidate-DNA helices. J Am Chem Soc. 1998;120:9401–9409.
- Kuhn B, Kollman PA. Binding of a diverse set of ligands to avidin and streptavidin: An accurate quantitative prediction of their relative affinities by a combination of molecular mechanics and continuum solvent models. J Med Chem. 2000;43:3786–3791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM63592/GM/NIGMS NIH HHS/United States
- R01 GM059957/GM/NIGMS NIH HHS/United States
- GM59957/GM/NIGMS NIH HHS/United States
- R01 GM034993/GM/NIGMS NIH HHS/United States
- R01 GM063592/GM/NIGMS NIH HHS/United States
- R56 GM063592/GM/NIGMS NIH HHS/United States