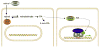Retinoic acid regulation of the somitogenesis clock - PubMed (original) (raw)
Review
Retinoic acid regulation of the somitogenesis clock
Gregg Duester. Birth Defects Res C Embryo Today. 2007 Jun.
Abstract
Retinoic acid (RA) is a signaling molecule synthesized from vitamin A that controls gene expression at the transcriptional level by functioning as a ligand for nuclear RA receptors. RA plays an essential role during embryonic development in higher animals by regulating key genes involved in pattern formation. RA is required for induction of several Hox genes involved in patterning of the hindbrain and spinal cord as neuroectoderm emerges from the primitive streak. Recent findings indicate that RA is also required to ensure bilaterally symmetrical generation of left and right somites as presomitic mesoderm emerges from the primitive streak. RA may control somitogenesis through its ability to repress posterior ectodermal expression of fibroblast growth factor-8 (Fgf8) for a short period of time during the late primitive streak stage when the somitogenesis clock initiates. During this tight temporal window, RA is required to limit Fgf8 expression to the most posterior ectoderm (epiblast), thus preventing ectopic Fgf8 expression in more anterior ectoderm including the node ectoderm and neuroectoderm. Although Fgf8 is required for the node to impart left-right asymmetry on specific tissues (heart, visceral organs, etc.), excess Fgf8 signaling following a loss of RA may stimulate the node to generate asymmetry also in presomitic mesoderm, leading to left-right asymmetry in the somitogenesis clock. These findings suggest that human vertebral birth defects such as scoliosis, an abnormal left-right bending of the vertebral column, may be caused by a defect in RA signaling during somitogenesis.
(c) 2007 Wiley-Liss, Inc.
Figures
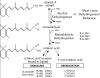
Figure 1
RA synthesis pathway. Conversion of vitamin A (retinol) to retinoic acid (RA) requires two sequential oxidation steps. First, retinol is oxidized to retinaldehyde by either alcohol dehydrogenase (ADH) or short-chain dehydrogenase/reductase (SDR). Second, retinaldehyde is oxidized to RA by retinaldehyde dehydrogenase (RALDH). Once synthesized, RA can regulate transcription by serving as a ligand for nuclear RA receptors (RAR), or RA can be further oxidized by P450 enzymes (CYP26) as part of a degradative pathway.
Figure 2
Control of RA signaling by retinoid-binding proteins. Retinol is carried in the bloodstream by serum retinol-binding protein (RBP), which facilitates entry of retinol into cells via a specific receptor. Upon entry, retinol binds cellular RBP (CRBP), which provides a sequestration function. Free retinol is oxidized to RA, some of which can diffuse out of the cell and enter nearby cells by passive diffusion facilitated by cellular RA-binding protein (CRABP), which provides a sequestration function. CRABP can also facilitate transport of RA to the nucleus, and upon dissociation from CRABP, RA can then bind the RA receptor (RAR). In the liganded state, RAR, bound to DNA as a heterodimer with RXR, recruits coactivator proteins that stimulate transcription. The DNA element to which RAR-RXR heterodimers bind is known as a retinoic acid response element (RARE).
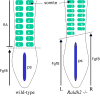
Figure 3
Disruption of somite left–right patterning in the absence of RA signaling. Depicted are embryos at the eight-somite stage in which the wild-type embryo has eight pairs of somites whereas the _Raldh2_−/− embryo has eight somites on the left side but only seven somites on the right. Thus, a loss of RA synthesis results in a loss of left–right bilateral symmetry during somite generation; plus, somites are smaller in size along the anteroposterior axis. RA is normally synthesized by Raldh2 expressed in the somites and RA released by somitic mesoderm functions to limit the anterior border of Fgf8 expression in ectodermal epiblast cells along the primitive streak (ps). Somites form in mesoderm just anterior to the Fgf8 expression domain. A loss of RA signaling results in an anterior advance of FGF8 signaling (advancing further on the right than on the left), which may be responsible for both the smaller size of somites and a failure to generate left–right somite pairs in synchrony.
Figure 4
Anterior advance of Fgf8 expression following a loss of RA signaling. Fgf8 expression is normally confined to primitive streak (ps) tissues posterior to the node, a structure that plays a role in gastrulation and left–right patterning of lateral plate mesoderm. RA is first generated by Raldh2 expressed in the presomitic mesoderm (psm) during the late primitive streak stage, when it is required to limit the border of Fgf8 expression to a position just posterior to the node at the anterior end of the primitive streak. A loss of RA allows Fgf8 expression to enter the node and adjacent neuroectoderm (ne), which may lead to excessive FGF8 signaling to mesoderm. Excessive FGF8 signaling in the presomitic mesoderm could result in less cells condensing to form each somite (smaller somites), and excessive FGF8 signaling in the node could impart left–right asymmetry on presomitic mesoderm, which normally does not exhibit such asymmetry.
Similar articles
- Retinoic Acid Activity in Undifferentiated Neural Progenitors Is Sufficient to Fulfill Its Role in Restricting Fgf8 Expression for Somitogenesis.
Cunningham TJ, Brade T, Sandell LL, Lewandoski M, Trainor PA, Colas A, Mercola M, Duester G. Cunningham TJ, et al. PLoS One. 2015 Sep 14;10(9):e0137894. doi: 10.1371/journal.pone.0137894. eCollection 2015. PLoS One. 2015. PMID: 26368825 Free PMC article. - Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm.
Sirbu IO, Duester G. Sirbu IO, et al. Nat Cell Biol. 2006 Mar;8(3):271-7. doi: 10.1038/ncb1374. Epub 2006 Feb 19. Nat Cell Biol. 2006. PMID: 16489341 Free PMC article. - Retinoic acid controls body axis extension by directly repressing Fgf8 transcription.
Kumar S, Duester G. Kumar S, et al. Development. 2014 Aug;141(15):2972-7. doi: 10.1242/dev.112367. Development. 2014. PMID: 25053430 Free PMC article. - Genetic analysis of molecular oscillators in mammalian somitogenesis: clues for studies of human vertebral disorders.
Sewell W, Kusumi K. Sewell W, et al. Birth Defects Res C Embryo Today. 2007 Jun;81(2):111-20. doi: 10.1002/bdrc.20091. Birth Defects Res C Embryo Today. 2007. PMID: 17600783 Review. - Somitogenesis.
Gossler A, Hrabĕ de Angelis M. Gossler A, et al. Curr Top Dev Biol. 1998;38:225-87. Curr Top Dev Biol. 1998. PMID: 9399080 Review.
Cited by
- Comparative analysis of serum proteome in congenital scoliosis patients with TBX6 haploinsufficiency - a first report pointing to lipid metabolism.
Zhu Q, Wu N, Liu G, Zhou Y, Liu S, Chen J, Liu J, Zuo Y, Liu Z, Chen W, Chen Y, Chen J, Lin M, Zhao Y, Yang Y, Wang S, Yang X, Ma Y, Wang J, Chen X, Zhang J, Shen J, Wu Z, Qiu G. Zhu Q, et al. J Cell Mol Med. 2018 Jan;22(1):533-545. doi: 10.1111/jcmm.13341. Epub 2017 Sep 25. J Cell Mol Med. 2018. PMID: 28944995 Free PMC article. - Growth differentiation factor 11 signaling controls retinoic acid activity for axial vertebral development.
Lee YJ, McPherron A, Choe S, Sakai Y, Chandraratna RA, Lee SJ, Oh SP. Lee YJ, et al. Dev Biol. 2010 Nov 1;347(1):195-203. doi: 10.1016/j.ydbio.2010.08.022. Epub 2010 Aug 27. Dev Biol. 2010. PMID: 20801112 Free PMC article. - Retinoic acid actions through mammalian nuclear receptors.
Huang P, Chandra V, Rastinejad F. Huang P, et al. Chem Rev. 2014 Jan 8;114(1):233-54. doi: 10.1021/cr400161b. Epub 2013 Dec 5. Chem Rev. 2014. PMID: 24308533 Free PMC article. Review. No abstract available. - Congenital scoliosis in Smith-Magenis syndrome: a case report and review of the literature.
Li Z, Shen J, Liang J, Sheng L. Li Z, et al. Medicine (Baltimore). 2015 May;94(17):e705. doi: 10.1097/MD.0000000000000705. Medicine (Baltimore). 2015. PMID: 25929900 Free PMC article. Review. - The expanding role for retinoid signaling in heart development.
Hoover LL, Burton EG, Brooks BA, Kubalak SW. Hoover LL, et al. ScientificWorldJournal. 2008 Feb 25;8:194-211. doi: 10.1100/tsw.2008.39. ScientificWorldJournal. 2008. PMID: 18661045 Free PMC article. Review.
References
- Ang HL, Deltour L, Hayamizu TF, et al. Retinoic acid synthesis in mouse embryos during gastrulation and craniofacial development linked to class IV alcohol dehydrogenase gene expression. J Biol Chem. 1996;271:9526–9534. - PubMed
- Aulehla A, Herrmann BG. Segmentation in vertebrates: clock and gradient finally joined. Genes Dev. 2004;18:2060–2067. - PubMed
- Aulehla A, Wehrle C, Brand-Saberi B, et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. - PubMed
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. - PubMed
- Balmer JE, Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J Steroid Biochem Mol Biol. 2005;96:347–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources