Regulation of the bioavailability of thioredoxin in the lens by a specific thioredoxin-binding protein (TBP-2) - PubMed (original) (raw)
Regulation of the bioavailability of thioredoxin in the lens by a specific thioredoxin-binding protein (TBP-2)
Namal P M Liyanage et al. Exp Eye Res. 2007 Aug.
Abstract
Thioredoxin (TRx) is known to control redox homeostasis in cells. In recent years, a specific TRx binding protein called thioredoxin binding protein-2 (TBP-2) was found in other cell types and it appeared to negatively regulate TRx bioavailability and thereby control TRx biological function. In view of the sensitivity of lens transparency to redox status, proper regulation of TRx bioavailability is of the utmost importance. This study was conducted to examine the presence and function of TBP-2 in human lens epithelial cells (HLE B3). We cloned human lens TBP-2 from a human cDNA library (GenBank accession number AY 594328) and showed that it is fully homologous to the human brain TBP-2 gene. The recombinant TBP-2 protein was partially purified and mass spectrometric analysis confirmed its sequence homology to that of brain TBP-2. Immunoprecipitates obtained from HLE B3 cells using anti-TRx and anti-TBP-2 antibodies showed the presence of TRx and TBP-2 in immunoprecipitates indicating the formation of a TRx-TBP-2 complex in vivo. Furthermore, under H(2)O(2)-stress conditions, TRx gene expression was transiently up-regulated while TBP-2 gene expression was inversely down-regulated as seen in both HLE B3 cells and in the epithelial cell layers from cultured pig lenses. Cells with overexpressed TBP-2 showed lower TRx activity, grew slower and were more susceptible to oxidative stress-induced apoptosis. This is the first report of the presence of a TRx-specific binding protein in the lens. Our data suggest that TBP-2 is likely a negative regulator for the bioavailability, and therefore, the overall function of TRx in the lens.
Figures
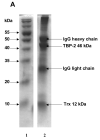
Figure 1. Immunoprecipitation of TBP-2-TRx complex by anti-TRx and anti-TBP-2 antibodies
HLE B3 cell lysate was incubated with either anti-TRx or anti-TBP-2 antibody. Protein-A Agarose was added and incubated as described in the Methods. Immunoprecipitate was then collected by centrifugation. A, SDS-PAGE analysis of immunoprecipitate obtained using anti-TRx antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. B, SDS-PAGE of immunoprecipitate with anti-TBP-2 antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. C, Western blot of the immunoprecipitate obtained with anti-TRx antibody. D, Western blot analysis of the immunoprecipitate obtained with anti-TBP-2 antibody. For western blot analysis immunoprecipitation was done using Seize® X Protein A Immunoprecipitation kit (PIERCE, IL).
Figure 1. Immunoprecipitation of TBP-2-TRx complex by anti-TRx and anti-TBP-2 antibodies
HLE B3 cell lysate was incubated with either anti-TRx or anti-TBP-2 antibody. Protein-A Agarose was added and incubated as described in the Methods. Immunoprecipitate was then collected by centrifugation. A, SDS-PAGE analysis of immunoprecipitate obtained using anti-TRx antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. B, SDS-PAGE of immunoprecipitate with anti-TBP-2 antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. C, Western blot of the immunoprecipitate obtained with anti-TRx antibody. D, Western blot analysis of the immunoprecipitate obtained with anti-TBP-2 antibody. For western blot analysis immunoprecipitation was done using Seize® X Protein A Immunoprecipitation kit (PIERCE, IL).
Figure 1. Immunoprecipitation of TBP-2-TRx complex by anti-TRx and anti-TBP-2 antibodies
HLE B3 cell lysate was incubated with either anti-TRx or anti-TBP-2 antibody. Protein-A Agarose was added and incubated as described in the Methods. Immunoprecipitate was then collected by centrifugation. A, SDS-PAGE analysis of immunoprecipitate obtained using anti-TRx antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. B, SDS-PAGE of immunoprecipitate with anti-TBP-2 antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. C, Western blot of the immunoprecipitate obtained with anti-TRx antibody. D, Western blot analysis of the immunoprecipitate obtained with anti-TBP-2 antibody. For western blot analysis immunoprecipitation was done using Seize® X Protein A Immunoprecipitation kit (PIERCE, IL).
Figure 2. The effect of a 0.1 mM bolus of H2O2 on TRx and TBP-2 protein and mRNA expression in HLE B3 cells
A, Western blot analysis of cell lysates. HLE B3 cells were treated with H2O2 for indicated time points. Then cells were lysed and subjected to SDS-PAGE (40 μg of protein). The separated proteins were transferred to a nitrocellulose membrane and probed with antibody specific to TBP-2, TRx and β-actin (internal control). Immune complexes were visualized with the use of suitable secondary antibodies. B, Real-time PCR quantification of TRx and TBP-2 mRNA expression. HLE B3 cells were treated with a 0.1 mM bolus of H2O2 for indicated times, cells collected at each time point, mRNA extracted and reverse transcribed as described in methods section. Quantification of mRNA for TRx (◆) and TBP-2 (■) was carried out by real-time PCR as a function of time as described. β-actin was used as an internal control. Data analysis was done by ΔΔCt method. The results are based on the average of 4 determinations. Error bars indicate standard errors of the mean.
Figure 3. The effect of H2O2 on TRx and TBP-2 mRNA and protein expression in the epithelium of cultured pig lens
Fresh pig lenses were incubated in TC199 medium with or without 0.2 mM H2O2 for 24hours. The concentration of H2O2 was maintained using 2.31 unit of glucose oxidase in the medium. The lenses were taken at the indicated time points and the epithelial layers were removed. A, Effect of H2O2 on protein expression. The total soluble protein fraction was prepared and 40μg of protein from each time point was subjected to immunoblot analysis with antibodies specific to TRx and TBP-2. Glyceraldehyde-3-phosphate dehydrogenase (G3PD) was used as an internal control. B, Effect of H2O2 on mRNA expression. Lenses were removed at the indicated times and epithelial layers separated. Total RNA was extracted from the three pooled epithelial layers (in each group) and reverse transcribed. Primers were designed and synthesized to detect TBP-2, TRx and β-actin. Equal amounts of synthesized cDNA were amplified by PCR with synthesized primers. PCR products were analyzed by agarose gel electrophoresis. β-actin was amplified as an internal control.
Figure 3. The effect of H2O2 on TRx and TBP-2 mRNA and protein expression in the epithelium of cultured pig lens
Fresh pig lenses were incubated in TC199 medium with or without 0.2 mM H2O2 for 24hours. The concentration of H2O2 was maintained using 2.31 unit of glucose oxidase in the medium. The lenses were taken at the indicated time points and the epithelial layers were removed. A, Effect of H2O2 on protein expression. The total soluble protein fraction was prepared and 40μg of protein from each time point was subjected to immunoblot analysis with antibodies specific to TRx and TBP-2. Glyceraldehyde-3-phosphate dehydrogenase (G3PD) was used as an internal control. B, Effect of H2O2 on mRNA expression. Lenses were removed at the indicated times and epithelial layers separated. Total RNA was extracted from the three pooled epithelial layers (in each group) and reverse transcribed. Primers were designed and synthesized to detect TBP-2, TRx and β-actin. Equal amounts of synthesized cDNA were amplified by PCR with synthesized primers. PCR products were analyzed by agarose gel electrophoresis. β-actin was amplified as an internal control.
Figure 4. The effect of TBP-2 over-expression on HLE B3 cells
A, Western blot analysis of TBP-2 expression in control HLE B3 lysate (lane 1) and TBP-2 over-expressed HLE B3 lysate (lane 2). B, Comparison of the TRx activity in control and TBP-2 over-expressed HLE B3 cells. Cells from both groups (2 × 106 cells from each group) were lysed and TRx activity determined as described. C, Comparison of growth rates of control (●) and TBP-2 over-expressed (■) cells during the 4-day growth period by cell counting. The results are based on the average of 3 determinations. Error bars indicate standard errors of the mean. D, Effect of TBP-2 over-expression on H2O2 mediated apoptosis. Control vector only cells and TBP-2 over-expressed cells (OE) were treated either with serum free MEM or with 100 μM H2O2 for 1 hr. Then the cells were washed with PBS and medium was replaced with 20% serum containing medium and incubated at 37°C for 16 hrs. Cells were trypsinized, washed with PBS, stained with Annexin V-FITC and analyzed by flow cytometry (for details see Materials and Methods). The results are based on the average of 3 determinations. Error bars indicate standard errors of the mean.
Figure 4. The effect of TBP-2 over-expression on HLE B3 cells
A, Western blot analysis of TBP-2 expression in control HLE B3 lysate (lane 1) and TBP-2 over-expressed HLE B3 lysate (lane 2). B, Comparison of the TRx activity in control and TBP-2 over-expressed HLE B3 cells. Cells from both groups (2 × 106 cells from each group) were lysed and TRx activity determined as described. C, Comparison of growth rates of control (●) and TBP-2 over-expressed (■) cells during the 4-day growth period by cell counting. The results are based on the average of 3 determinations. Error bars indicate standard errors of the mean. D, Effect of TBP-2 over-expression on H2O2 mediated apoptosis. Control vector only cells and TBP-2 over-expressed cells (OE) were treated either with serum free MEM or with 100 μM H2O2 for 1 hr. Then the cells were washed with PBS and medium was replaced with 20% serum containing medium and incubated at 37°C for 16 hrs. Cells were trypsinized, washed with PBS, stained with Annexin V-FITC and analyzed by flow cytometry (for details see Materials and Methods). The results are based on the average of 3 determinations. Error bars indicate standard errors of the mean.
Similar articles
- Human lens thioredoxin: molecular cloning and functional characterization.
Yegorova S, Liu A, Lou MF. Yegorova S, et al. Invest Ophthalmol Vis Sci. 2003 Aug;44(8):3263-71. doi: 10.1167/iovs.02-1322. Invest Ophthalmol Vis Sci. 2003. PMID: 12882768 - Overexpression of thioredoxin-binding protein 2 increases oxidation sensitivity and apoptosis in human lens epithelial cells.
Yu Y, Xing K, Badamas R, Kuszynski CA, Wu H, Lou MF. Yu Y, et al. Free Radic Biol Med. 2013 Apr;57:92-104. doi: 10.1016/j.freeradbiomed.2012.12.022. Epub 2013 Jan 4. Free Radic Biol Med. 2013. PMID: 23291592 Free PMC article. - Induction of thioltransferase and thioredoxin/thioredoxin reductase systems in cultured porcine lenses under oxidative stress.
Moon S, Fernando MR, Lou MF. Moon S, et al. Invest Ophthalmol Vis Sci. 2005 Oct;46(10):3783-9. doi: 10.1167/iovs.05-0237. Invest Ophthalmol Vis Sci. 2005. PMID: 16186363 - Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2.
Watanabe R, Nakamura H, Masutani H, Yodoi J. Watanabe R, et al. Pharmacol Ther. 2010 Sep;127(3):261-70. doi: 10.1016/j.pharmthera.2010.04.004. Epub 2010 May 8. Pharmacol Ther. 2010. PMID: 20435060 Review. - Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome.
Kaimul AM, Nakamura H, Masutani H, Yodoi J. Kaimul AM, et al. Free Radic Biol Med. 2007 Sep 15;43(6):861-8. doi: 10.1016/j.freeradbiomed.2007.05.032. Epub 2007 Jun 6. Free Radic Biol Med. 2007. PMID: 17697931 Review.
Cited by
- Glutathione and Glutaredoxin in Redox Regulation and Cell Signaling of the Lens.
Lou MF. Lou MF. Antioxidants (Basel). 2022 Oct 1;11(10):1973. doi: 10.3390/antiox11101973. Antioxidants (Basel). 2022. PMID: 36290696 Free PMC article. Review. - The Potential Role of Gut Microbiota in the Pathogenesis of Type 2 Diabetes Mellitus via Epigenetics and Inflammasome.
Sharma B, Kumar A, Sharma U, Pal D, Prashar S. Sharma B, et al. Endocr Metab Immune Disord Drug Targets. 2022;22(14):1331-1343. doi: 10.2174/1871530322666220331152809. Endocr Metab Immune Disord Drug Targets. 2022. PMID: 35362379 Review. - MiRNAs regulate oxidative stress related genes via binding to the 3' UTR and TATA-box regions: a new hypothesis for cataract pathogenesis.
Wu C, Liu Z, Ma L, Pei C, Qin L, Gao N, Li J, Yin Y. Wu C, et al. BMC Ophthalmol. 2017 Aug 14;17(1):142. doi: 10.1186/s12886-017-0537-9. BMC Ophthalmol. 2017. PMID: 28806956 Free PMC article. - Autophagy and Age-Related Eye Diseases.
Yang X, Pan X, Zhao X, Luo J, Xu M, Bai D, Hu Y, Liu X, Yu Q, Gao D. Yang X, et al. Biomed Res Int. 2019 Dec 14;2019:5763658. doi: 10.1155/2019/5763658. eCollection 2019. Biomed Res Int. 2019. PMID: 31950044 Free PMC article. Review.
References
- Akamatsu Y, Ohno T, Hirota K, Kagoshima H, Yodoi J, Shigesada K. Redox regulation of the DNA binding activity in transcription factor PEBP2. The roles of two conserved cysteine residues. J. Biol. Chem. 1997;272:14497–14500. - PubMed
- Andley UP, Rhim JS, Chylack LT, Jr., Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994;35:3094–3102. - PubMed
- Bhuyan KC, Reddy PG, Bhuyan DK. Thioredoxin genes in lens: regulation by oxidative stress. Methods Enzymol. 2002;347:421–435. - PubMed
- Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219:26–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases