Shs1 plays separable roles in septin organization and cytokinesis in Saccharomyces cerevisiae - PubMed (original) (raw)
Shs1 plays separable roles in septin organization and cytokinesis in Saccharomyces cerevisiae
Masayuki Iwase et al. Genetics. 2007 Sep.
Abstract
In Saccharomyces cerevisiae, five septins (Cdc3, Cdc10, Cdc11, Cdc12, and Shs1/Sep7) form the septin ring at the bud neck during vegetative growth. We show here that disruption of SHS1 caused cold-sensitive growth in the W303 background, with cells arrested in chains, indicative of a cytokinesis defect. Surprisingly, the other four septins appeared to form an apparently normal septin ring in shs1Delta cells grown under the restrictive condition. We found that Myo1 and Iqg1, two components of the actomyosin contractile ring, and Cyk3, a component of the septum formation, were either delocalized or mislocalized in shs1Delta cells, suggesting that Shs1 plays supportive roles in cytokinesis. We also found that deletion of SHS1 enhanced or suppressed the septin defect in cdc10Delta and cdc11Delta cells, respectively, suggesting that Shs1 is involved in septin organization, exerting different effects on septin-ring assembly, depending on the composition of the septin subunits. Furthermore, we constructed an shs1-100c allele that lacks the coding sequence for the C-terminal 32 amino acids. This allele still displayed the genetic interactions with the septin mutants, but did not show cytokinesis defects as described above, suggesting that the roles of Shs1 in septin organization and cytokinesis are separable.
Figures
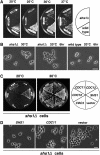
Figure 1.—
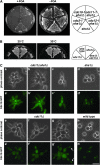
Phenotype of an _shs1_Δ disruptant. (A) Disruption of SHS1. One of the alleles of SHS1 in W303a/α was replaced with the shs1_Δ_∷HIS3 gene (Masa1). The resulting heterozygous diploid cells were sporulated and dissected. One wild-type and two disruptant segregants were chosen among the segregants and streaked across YPD plates, each of which was incubated at 20° for 4 days, or at 25°, 30°, and 37° for 2 days. (B) Cell morphology of _shs1_Δ cells. The _shs1_Δ cells (Masa2) and wild-type cells (W303a) were cultivated at 30° to midlogarithmic phase and then shifted to 20°. The cultures were incubated for another 6 hr. Bar, 10 μm. (C) Suppression of _shs1_Δ cells by a different septin gene on a multicopy vector. The indicated plasmid (CDC3, pM-9; CDC10, pM-10; CDC11, pM-11; CDC12, pM-12; SHS1, pM-13) was introduced into the _shs1_Δ cells (Masa2). One representative was selected from each transformation experiment and streaked on two SC–Ura plates, one of which was incubated at 20° for 4 days (left) and the other at 30° for 2 days (right). (D) Cell morphology of _shs1_Δ cells carrying CDC11 on a multicopy vector. The indicated plasmid (SHS1, pM-13; CDC11, pM-11; vector, pYO326) was introduced into the _shs1_Δ cells (Masa2). Cells were cultivated at 30° to midlogarithmic phase and then shifted to 20°. The cultures were incubated for another 6 hr. Bar, 10 μm.
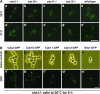
Figure 2.—
Localization of septin-GFPs in a septin mutant. (A) Localization of Shs1_-_GFP. The chromosomal SHS1 gene of each of the septin mutants was replaced with the SHS1-GFP gene. Cells of cdc3-1 SHS1-GFP (Masa11, a and a′), cdc10-1 SHS1-GFP (Masa12, b and b′), cdc11-1 SHS1-GFP (Masa13, c and c′), cdc12-1 SHS1-GFP (Masa14, d and d′), and SHS1-GFP (Masa6, e and e′) were grown at 25° to midlogarithmic phase and a part of each culture was shifted to 37°. After 4 hr of incubation at 37°, cells were harvested, fixed with 5% formaldehyde, and GFP was observed. Bar, 10 μm. (B) Localization of septin-GFPs in the _shs1_Δ strain. Septin-GFP fusion genes were separately introduced into the _shs1_Δ cells (Masa2). The _shs1_Δ cells expressing one of septin-GFP fusion protein were cultivated at 30° to midlogarithmic phase and then shifted to 20°. Cells were harvested at 6 hr after the shift, and GFP was observed. Bar, 10 μm. (a and a′) Cdc3-GFP (pM-1); (b and b′) Cdc10-GFP (pM-2); (c and c′) Cdc11-GFP (pM-3); (d and d′) Cdc12-GFP (pM-4); (e and e′) Masa6.
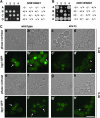
Figure 3.—
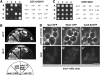
The interactions between the septins and cytokinesis genes. (A) The double mutant between shs1 and iqg1 was lethal. Tetrad analysis of diploid cells from a cross between _shs1_Δ cells (Masa2) and iqg1_Δ_∷IQG1-GFP cells (Masa98) was carried out. Tetrads were dissected and grown on YPD plate at 25° for 7 days. Genotypes of the segregants are indicated on the right. +, SHS1 or IQG1; −, _shs1_Δ or iqg1_Δ_∷IQG1-GFP. (B) The double mutant between cdc10 and iqg1 was viable. Tetrad analysis of diploid cells from a cross between _cdc10_Δ cells (Masa22) and iqg1_Δ_∷IQG1-GFP cells (Masa98) was carried out. Tetrads were dissected and grown on YPD plate at 25° for 9 days. Genotypes of the segregants are indicated on the right. +, CDC10 or IQG1; −, _cdc10_Δ or iqg1_Δ_∷IQG1-GFP. (C) The localization of Iqg1-GFP in an _shs1_Δ disruptant. The IQG1-GFP fusion gene (IQG1pUG35) was introduced into the _shs1_Δ cells (Masa2) and wild-type cells (W303a). Cells were cultivated at 30° to midlogarithmic phase and then shifted to 20°. Cells were harvested at 6 hr after the shift, and GFP was observed. Arrows indicate Iqg1-GFPs, and arrowheads indicate mislocalized Iqg1-GFPs. Bar, 10 μm. (a, a′, b, and b′) Wild type at 20°; (c, c′, d, and d′) _shs1_Δ at 20°; (e, e′, f, and f′) wild type at 30°; (g, g′, h, and h′) _shs1_Δ at 30°. (D) The localization of Myo1-GFP in an _shs1_Δ disruptant. The MYO1-GFP fusion gene was integrated into _shs1_Δ cells (Masa2) and wild-type cells (W303a). Cells were cultivated and observed as described in C. Arrows indicate Myo1-GFPs, and an arrowhead indicates mislocalized Myo1-GFP. Bar, 10 μm. (a, a′, b, and b′) Wild type (Masa105) at 20°; (c, c′, d, and d′) _shs1_Δ (Masa104) at 20°; (e, e′, f, and f′) wild type (Masa105) at 30°; (g, g′, h, and h′) _shs1_Δ (Masa104) at 30°. (E) The localization of Cyk3-8xGFP in an _shs1_Δ disruptant. The CYK3-8xGFP cells (YAT3200) were crossed with the _shs1_Δ cells (Masa2), and the resulting diploid cells were sporulated and dissected. _shs1_Δ cells containing CYK3-8xGFP and YAT3200 cells were cultivated and observed as described in C. Arrows indicate Cyk3-8xGFPs, and an arrowhead indicates mislocalized Cyk3-8xGFP. Bar, 10 μm. (a, a′, b, and b′) Wild type (YAT3200) at 20°; (c, c′, d, and d′) _shs1_Δ (Masa2175) at 20°; (e, e′, f, and f′) wild type (YAT3200) at 30°; (g, g′, h, and h′) _shs1_Δ (Masa2175) at 30°.
Figure 3.—
The interactions between the septins and cytokinesis genes. (A) The double mutant between shs1 and iqg1 was lethal. Tetrad analysis of diploid cells from a cross between _shs1_Δ cells (Masa2) and iqg1_Δ_∷IQG1-GFP cells (Masa98) was carried out. Tetrads were dissected and grown on YPD plate at 25° for 7 days. Genotypes of the segregants are indicated on the right. +, SHS1 or IQG1; −, _shs1_Δ or iqg1_Δ_∷IQG1-GFP. (B) The double mutant between cdc10 and iqg1 was viable. Tetrad analysis of diploid cells from a cross between _cdc10_Δ cells (Masa22) and iqg1_Δ_∷IQG1-GFP cells (Masa98) was carried out. Tetrads were dissected and grown on YPD plate at 25° for 9 days. Genotypes of the segregants are indicated on the right. +, CDC10 or IQG1; −, _cdc10_Δ or iqg1_Δ_∷IQG1-GFP. (C) The localization of Iqg1-GFP in an _shs1_Δ disruptant. The IQG1-GFP fusion gene (IQG1pUG35) was introduced into the _shs1_Δ cells (Masa2) and wild-type cells (W303a). Cells were cultivated at 30° to midlogarithmic phase and then shifted to 20°. Cells were harvested at 6 hr after the shift, and GFP was observed. Arrows indicate Iqg1-GFPs, and arrowheads indicate mislocalized Iqg1-GFPs. Bar, 10 μm. (a, a′, b, and b′) Wild type at 20°; (c, c′, d, and d′) _shs1_Δ at 20°; (e, e′, f, and f′) wild type at 30°; (g, g′, h, and h′) _shs1_Δ at 30°. (D) The localization of Myo1-GFP in an _shs1_Δ disruptant. The MYO1-GFP fusion gene was integrated into _shs1_Δ cells (Masa2) and wild-type cells (W303a). Cells were cultivated and observed as described in C. Arrows indicate Myo1-GFPs, and an arrowhead indicates mislocalized Myo1-GFP. Bar, 10 μm. (a, a′, b, and b′) Wild type (Masa105) at 20°; (c, c′, d, and d′) _shs1_Δ (Masa104) at 20°; (e, e′, f, and f′) wild type (Masa105) at 30°; (g, g′, h, and h′) _shs1_Δ (Masa104) at 30°. (E) The localization of Cyk3-8xGFP in an _shs1_Δ disruptant. The CYK3-8xGFP cells (YAT3200) were crossed with the _shs1_Δ cells (Masa2), and the resulting diploid cells were sporulated and dissected. _shs1_Δ cells containing CYK3-8xGFP and YAT3200 cells were cultivated and observed as described in C. Arrows indicate Cyk3-8xGFPs, and an arrowhead indicates mislocalized Cyk3-8xGFP. Bar, 10 μm. (a, a′, b, and b′) Wild type (YAT3200) at 20°; (c, c′, d, and d′) _shs1_Δ (Masa2175) at 20°; (e, e′, f, and f′) wild type (YAT3200) at 30°; (g, g′, h, and h′) _shs1_Δ (Masa2175) at 30°.
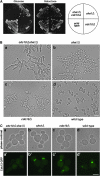
Figure 4.—
SHS1 was required for septin assembly in the _cdc10_Δ cells. (A) The _shs1_Δ _cdc10_Δ cells were lethal. The _cdc10_Δ cells (Masa24) were crossed with the _shs1_Δ cells (Masa2), and the resulting diploid cells (Masa31) containing GAL1-CDC10 plasmid (pM-92) were sporulated and dissected. A set of spore clones derived from one tetra-type ascus was streaked on YPD plates at 25° for 7 days and on YPGal plate at 25° for 4 days. _shs1_Δ _cdc10_Δ, Masa90; _shs1_Δ, Masa106; _cdc10_Δ, Masa102; wild type, Masa107. (B) The morphology of the _shs1_Δ _cdc10_Δ cells. The set of spore clones derived from one tetra-type ascus, which was used in (A) was grown to midlogarithmic phase at 25° in SCgal-Trp medium, and then harvested, washed, and resuspended into SC-Trp medium, where the expression of CDC10 was shut off, at 25°. After 8 hr of incubation, cells were harvested and observed. Bar, 10 μm. (a) _cdc10_Δ _shs1_Δ (Masa90); (b) _shs1_Δ (Masa106); (c) _cdc10_Δ (Masa102); (d) wild type (Masa107). (C) Septin-GFP was not localized at the bud neck in the _cdc10_Δ _shs1_Δ cells. The set of spore clones derived from one tetra-type ascus that was used in A was employed in this experiment. CDC12-GFP plasmid (pM-4) was introduced into each strain and grown to midlogarithmic phase at 25° in SCgal–Ura medium and then harvested and washed. Cells were resuspended into SC–Ura medium to shut off GAL1-CDC10 expression at 25°C. After 8 hr of incubation, cells were harvested and subjected to GFP observation. Bar, 5 μm. (a and a′) _cdc10_Δ _shs1_Δ (Masa90[pM-4]); (b and b′) _shs1_Δ (Masa106[pM-4]); (c and c′) _cdc10_Δ (Masa102[pM-4]); (d and d′) wild type (Masa107[pM-4]).
Figure 5.—
Interactions between SHS1 and CDC11. (A) Phenotypes of the double mutants. The shs1_Δ_∷HIS3 cells (Masa2) containing SHS1 on multicopy plasmid (pM-13) were crossed with each of the septin mutants (cdc3-1, YAT1775; cdc10-1, YAT1769; cdc11-1, YAT1773; cdc12-1, YAT1765). A double mutant containing pM-13 from each cross was selected and streaked across FOA plate (+FOA) and SC–Ura plate (−FOA), which were incubated at 25° for 4 days. _cdc3-1 shs1_Δ, Masa86; _cdc10-1 shs1_Δ, Masa87; _cdc11-1 shs1_Δ, Masa88; _cdc12-1 shs1_Δ, Masa89; _shs1_Δ, Masa103. (B) _cdc11_Δ was suppressed by _shs1_Δ. The _cdc11_Δ cells (Masa27) were crossed with the _shs1_Δ cells (Masa4), and the resulting diploid cells were sporulated and dissected. A set of spore clones derived from one tetra-type ascus was streaked on two YPD plates, one of which was incubated at 30° and the other at 25° for 2 days. (C) Cdc12-GFP localization in the _cdc11_Δ _shs1_Δ and _cdc11_Δ cells. Plasmid carrying the CDC12-GFP fusion gene (pM-4) was introduced into a set of tetrad segregants described in B. Each transformant was grown to midlogarithmic phase at 25° and then shifted to 30°. After 4 hr of incubation, cells were harvested and subjected to GFP observation. Arrows indicates cortical Cdc12-GFPs. Bar, 10 μm. (a, a′, b, b′, c, and c′) _cdc11_Δ _shs1_Δ; (d and d′) _shs1_Δ; (e, e′, f, f′, g, and g′) _cdc11_Δ; (h and h′) wild type. (D) Growth inhibition of _cdc11_Δ cells by overproduction of Shs1. GAL1-GFP-SHS1 plasmid (pM-91) and vector plasmid (pGAL-GFP) were separately introduced into the _cdc11_Δ cells (Masa27) and wild-type cells (YPH499). Two transformants from each transformation experiment were streaked on SC–Ura plate and SGal–Ura plate at 25° for 4 days. (E) The morphology of the _cdc11_Δ cells overproducing Shs1. A set of transformants used in D was grown to midlogarithmic phase at 25° in SCgal–URA medium for 15 hr and then observed. Bar, 10 μm. (a) _cdc11_Δ (Masa27[pM-91]); (b) _cdc11_Δ (Masa27[pGAL-GFP]); (c) wild type (YPH499[pM-91]); (d) wild type (YPH499[pGAL-GFP]).
Figure 5.—
Interactions between SHS1 and CDC11. (A) Phenotypes of the double mutants. The shs1_Δ_∷HIS3 cells (Masa2) containing SHS1 on multicopy plasmid (pM-13) were crossed with each of the septin mutants (cdc3-1, YAT1775; cdc10-1, YAT1769; cdc11-1, YAT1773; cdc12-1, YAT1765). A double mutant containing pM-13 from each cross was selected and streaked across FOA plate (+FOA) and SC–Ura plate (−FOA), which were incubated at 25° for 4 days. _cdc3-1 shs1_Δ, Masa86; _cdc10-1 shs1_Δ, Masa87; _cdc11-1 shs1_Δ, Masa88; _cdc12-1 shs1_Δ, Masa89; _shs1_Δ, Masa103. (B) _cdc11_Δ was suppressed by _shs1_Δ. The _cdc11_Δ cells (Masa27) were crossed with the _shs1_Δ cells (Masa4), and the resulting diploid cells were sporulated and dissected. A set of spore clones derived from one tetra-type ascus was streaked on two YPD plates, one of which was incubated at 30° and the other at 25° for 2 days. (C) Cdc12-GFP localization in the _cdc11_Δ _shs1_Δ and _cdc11_Δ cells. Plasmid carrying the CDC12-GFP fusion gene (pM-4) was introduced into a set of tetrad segregants described in B. Each transformant was grown to midlogarithmic phase at 25° and then shifted to 30°. After 4 hr of incubation, cells were harvested and subjected to GFP observation. Arrows indicates cortical Cdc12-GFPs. Bar, 10 μm. (a, a′, b, b′, c, and c′) _cdc11_Δ _shs1_Δ; (d and d′) _shs1_Δ; (e, e′, f, f′, g, and g′) _cdc11_Δ; (h and h′) wild type. (D) Growth inhibition of _cdc11_Δ cells by overproduction of Shs1. GAL1-GFP-SHS1 plasmid (pM-91) and vector plasmid (pGAL-GFP) were separately introduced into the _cdc11_Δ cells (Masa27) and wild-type cells (YPH499). Two transformants from each transformation experiment were streaked on SC–Ura plate and SGal–Ura plate at 25° for 4 days. (E) The morphology of the _cdc11_Δ cells overproducing Shs1. A set of transformants used in D was grown to midlogarithmic phase at 25° in SCgal–URA medium for 15 hr and then observed. Bar, 10 μm. (a) _cdc11_Δ (Masa27[pM-91]); (b) _cdc11_Δ (Masa27[pGAL-GFP]); (c) wild type (YPH499[pM-91]); (d) wild type (YPH499[pGAL-GFP]).
Figure 6.—
Phenotypes of the shs1-100c allele. (A) Two-hybrid interaction between Cdc12 and Shs1 truncated versions. Cells of J21 containing DBD-Cdc12 (pM-19) were transformed with AD-Shs1 (pM-93 (+1 aa to +90 aa), pM-94 (+91 aa to +318 aa), r26-5 (+312 aa to +551 aa), pM-95 (+1 aa to +253 aa), pM-2001 (+1 aa to +354 aa), pM-96 (+48 aa to +433 aa), pM-98 or pM109 (+1 aa to +519 aa), or pM-25 or pM-26 (+1 aa to +551 aa). The β-galactosidase activity of each transformant was measured. Shs1 derivatives that gave a higher β-galactosidase activity than a vector control were indicated as +, and those that gave a lower activity than a vector control were indicated as −. Each transformant was streaked on SC-Trp-Leu-His plate. +, growth; −, no growth. (B) Western blot analysis of AD-Shs1. Cell lysate prepared from the J21 cells carrying pM-25 (+1 aa to +551 aa), pM-109 (+1 aa to +519 aa), r26-5 (+312 aa to +551 aa), or pACT (vector plasmid) was subjected to electrophoresis on a 7.5% SDS–polyacrylamide gel followed by Western blot analysis using anti-GAL4 AD antibody (CLONTECH). Cdc28 was detected with anti-PSTAIRE antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as an internal reference. (C) Growth characteristics of the shs1-100c cells. _shs1_Δ cells (Masa2), shs1-100c cells (Masa91), and wild-type cells (W303a) were streaked across two YPD plates, each one of which was incubated at 30° for 3 days and the other at 18° for 4 days. (D) Localization of shs1-100c-GFP. SHS1–GFP (pM-101), shs1-100c-GFP (pM-102), and vector plasmids (pTS910CU) were separately introduced into the _shs1_Δ cells (Masa2). Cells were cultivated at 30° to midlogarithmic phase and then shifted to 20°. Cells were harvested at 6 hr after the shift, and GFP was observed. Bar, 5 μm. (a and a′) Shs1_–_GFP; (b and b′) shs1-100c-GFP; (c and c′) vector.
Figure 7.—
Phenotypes of the shs1-100c allele. (A) Genetic interaction with CDC10. Tetrad analysis of diploid cells from a cross between _cdc10_Δ cells (Masa24) and shs1-100c (Masa91) was carried out. Tetrads were dissected and grown on YPD plate at 25° for 4 days. Genotypes of the segregants are indicated in the right. +, CDC10 or SHS1. −, _cdc10_Δ or shs1-100c. (B) Genetic interaction with CDC11. The _cdc11_Δ cells (Masa27) were crossed with the shs1-100c cells (Masa93), and the resulting diploid cells were sporulated and dissected. A set of spore clones derived from one tetra-type ascus was streaked on two YPD plates, one of which was incubated at 30° and the other at 25° for 3 days. (C) Genetic interaction with IQG1. Tetrad analysis of diploid cells from a cross between iqg1_Δ_∷IQG1-GFP cells (Masa98) and shs1-100c (Masa91) was carried out. Tetrads were dissected and grown on YPD plate at 25° for 7 days. Genotypes of the segregants are indicated on the right. +, IQG1 or SHS1. −, iqg1_Δ_∷IQG1-GFP or shs1-100c. (D) The localization of Iqg1-GFP, Myo1-GFP, Cyk3-8xGFP in shs1-100c. The shs1-100c cells (Masa91) containing the IQG1-GFP fusion plasmid (IQG1pUG35), MYO1-GFP integrated shs1-100c cells (Masa2154), and CYK3-8xGFP integrated shs1-100c cells (Masa2187) were cultivated and observed as described in Figure 3, C–E. Bar, 5 μm. (a and a′) Iqg1-GFP; (b and b′) Myo1-GFP; (c and c′) Cyk3-8xGFP.
Figure 8.—
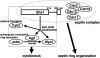
A model for the roles of Shs1 in septin organization and cytokinesis. Shs1 exerts different effects on septin organization depending on the subunit compositions of the septin complexes. Shs1 promotes and inhibits septin ring assembly in _cdc10_Δ and _cdc11_Δ cells, respectively. Shs1 lacking the C-terminal 32 amino acids can carry out its function in cytokinesis normally, including the recruitment of the components involved in the actomyosin contractile ring assembly such as Myo1 and Iqg1 as well as those involved in septum formation such as Cyk3. In contrast, the last 32 amino acids of Shs1 are required for its role in septin organization (see text for details).
Similar articles
- Comprehensive Genetic Analysis of Paralogous Terminal Septin Subunits Shs1 and Cdc11 in Saccharomyces cerevisiae.
Finnigan GC, Takagi J, Cho C, Thorner J. Finnigan GC, et al. Genetics. 2015 Jul;200(3):821-41. doi: 10.1534/genetics.115.176495. Epub 2015 May 12. Genetics. 2015. PMID: 25971665 Free PMC article. - Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae.
Versele M, Gullbrand B, Shulewitz MJ, Cid VJ, Bahmanyar S, Chen RE, Barth P, Alber T, Thorner J. Versele M, et al. Mol Biol Cell. 2004 Oct;15(10):4568-83. doi: 10.1091/mbc.e04-04-0330. Epub 2004 Jul 28. Mol Biol Cell. 2004. PMID: 15282341 Free PMC article. - Subunit-dependent modulation of septin assembly: budding yeast septin Shs1 promotes ring and gauze formation.
Garcia G 3rd, Bertin A, Li Z, Song Y, McMurray MA, Thorner J, Nogales E. Garcia G 3rd, et al. J Cell Biol. 2011 Dec 12;195(6):993-1004. doi: 10.1083/jcb.201107123. Epub 2011 Dec 5. J Cell Biol. 2011. PMID: 22144691 Free PMC article. - Septin mutations and phenotypes in S. cerevisiae.
Mela A, Momany M. Mela A, et al. Cytoskeleton (Hoboken). 2019 Jan;76(1):33-44. doi: 10.1002/cm.21492. Epub 2018 Nov 18. Cytoskeleton (Hoboken). 2019. PMID: 30171672 Review. - Diversity of septin scaffolds.
Kinoshita M. Kinoshita M. Curr Opin Cell Biol. 2006 Feb;18(1):54-60. doi: 10.1016/j.ceb.2005.12.005. Epub 2005 Dec 13. Curr Opin Cell Biol. 2006. PMID: 16356703 Review.
Cited by
- Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae.
Bertin A, McMurray MA, Pierson J, Thai L, McDonald KL, Zehr EA, García G 3rd, Peters P, Thorner J, Nogales E. Bertin A, et al. Mol Biol Cell. 2012 Feb;23(3):423-32. doi: 10.1091/mbc.E11-10-0850. Epub 2011 Dec 7. Mol Biol Cell. 2012. PMID: 22160597 Free PMC article. - ALIBY: ALFA Nanobody-Based Toolkit for Imaging and Biochemistry in Yeast.
Akhuli D, Dhar A, Viji AS, Bhojappa B, Palani S. Akhuli D, et al. mSphere. 2022 Oct 26;7(5):e0033322. doi: 10.1128/msphere.00333-22. Epub 2022 Oct 3. mSphere. 2022. PMID: 36190134 Free PMC article. - Cell polarization and cytokinesis in budding yeast.
Bi E, Park HO. Bi E, et al. Genetics. 2012 Jun;191(2):347-87. doi: 10.1534/genetics.111.132886. Genetics. 2012. PMID: 22701052 Free PMC article. Review. - Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly.
Bertin A, McMurray MA, Grob P, Park SS, Garcia G 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E. Bertin A, et al. Proc Natl Acad Sci U S A. 2008 Jun 17;105(24):8274-9. doi: 10.1073/pnas.0803330105. Epub 2008 Jun 12. Proc Natl Acad Sci U S A. 2008. PMID: 18550837 Free PMC article. - Septin-Associated Protein Kinases in the Yeast Saccharomyces cerevisiae.
Perez AM, Finnigan GC, Roelants FM, Thorner J. Perez AM, et al. Front Cell Dev Biol. 2016 Nov 1;4:119. doi: 10.3389/fcell.2016.00119. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27847804 Free PMC article. Review.
References
- Bi, E., 2001. Cytokinesis in budding yeast: the relationship between actomyosin ring function and septum formation. Cell Struct. Funct. 26: 529–537. - PubMed
- Bouquin, N., Y. Barral, R. Courbeyrette, M. Blondel, M. Snyder et al., 2000. Regulation of cytokinesis by the Elm1 protein kinase in Saccharomyces cerevisiae. J. Cell Sci. 113: 1435–1445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials