Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia - PubMed (original) (raw)
Comparative Study
. 2007 Aug;151(8):1377-84.
doi: 10.1038/sj.bjp.0707285. Epub 2007 Jul 2.
Affiliations
- PMID: 17603558
- PMCID: PMC2189829
- DOI: 10.1038/sj.bjp.0707285
Comparative Study
Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia
Y Wang et al. Br J Pharmacol. 2007 Aug.
Abstract
Background and purpose: Recombinant human erythropoietin (rhEPO; Epoetin-alpha; PROCRITtrade mark) has been shown to exert neuroprotective and restorative effects in a variety of CNS injury models. However, limited information is available regarding the dose levels required for these beneficial effects or the neuronal responses that may underlie them. Here we have investigated the dose-response to rhEPO and compared the effects of rhEPO with those of carbamylated rhEPO (CEPO) in a model of cerebral stroke in rats.
Experimental approach: Rats subjected to embolic middle cerebral artery occlusion (MCAo) were treated with rhEPO or CEPO, starting at 6 h and repeated at 24 and 48 h, after MCAo. Cerebral infarct volumes were assessed at 28 days and neurological impairment at 7, 14, 21 and 28 days, post-MCAo.
Key results: rhEPO at dose levels of 500, 1150 or 5000 IU kg(-1) or CEPO at a dose level of 50 microg kg(-1) significantly reduced cortical infarct volume and reduced neurologic impairment. All doses of rhEPO, but not CEPO, produced a transient increase in haematocrit, while rhEPO and CEPO substantially reduced the number of apoptotic cells and activated microglia in the ischemic boundary region.
Conclusions and implications: These data indicate that rhEPO and CEPO have anti-inflammatory and anti-apoptotic effects, even with administration at 6 h following embolic MCAo in rats. Taken together, these actions of rhEPO and CEPO are likely to contribute to their reduction of neurologic impairment following cerebral ischemia.
Figures
Figure 1
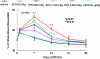
Changes in haematocrit before, during and after treatment with rhEPO and CEPO. Zero and 1 day time points represent prior to MCA occlusion and CEPO or rhEPO treatment, respectively. *P<0.05 and **P<0.01 vs the vehicle group. _N_=10 rats per group. CEPO, carbamylated rhEPO; MCA, middle cerebral artery; rhEPO, recombinant human erythropoietin.
Figure 2
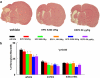
Infarct volumes 28 days after embolic MCA occlusion. Panel a shows infarction on a coronal section stained with H&E of a representative rat from vehicle, rhEPO 5000 IU kg−1 and CEPO 50 _μ_g kg−1 groups. Panel b shows quantitative analysis revealing that delayed (6 h) treatment with CEPO or rhEPO reduced infarct volume. Infarct volumes were measured as a whole hemisphere (Whole), cortex and subcortex. _N_=12 rats for control, rhEPO 500 IU kg−1, and rhEPO 5000 IU kg−1 groups. _N_=6 rats for rhEPO 50 IU kg−1, rhEPO 1150 IU kg−1, and CEPO 50 _μ_g kg−1 groups. *P<0.05 vs the vehicle group. CEPO, carbamylated rhEPO; H&E, haematoxylin and eosin; MCA, middle cerebral artery; rhEPO, recombinant human erythropoietin.
Figure 3
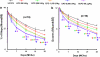
The effects of CEPO and rhEPO on neurological function. Delayed (6 h) treatment with CEPO or rhEPO improves neurological function measured by foot-fault test (a) and mNSS (b) compared with the vehicle group. *P<0.05 vs the vehicle group and +P<0.05 vs rhEPO 500 and 1150 IU kg−1 groups. _N_=10 rats per group. CEPO, carbamylated rhEPO; mNSS, modified neurological severity score; rhEPO, recombinant human erythropoietin.
Figure 4
The effect of CEPO and EPO on apoptosis and microglial responses. Panels a–f are images of activated microglial cells identified by IB4-positive cells in the cortical boundary region from representative rats treated with vehicle (a and b), rhEPO 5000 IU kg−1 (c and d) or CEPO 50 _μ_g kg−1 (e and f). Panels b, d and f are high magnification images from the box area in panels a, c and e, respectively. (g and h) Quantitative data of TUNEL-positive cells and activated microglial cells, respectively, in the ischaemic boundary region. Core in the panels a, c and e indicates the ischaemic core. Bar=80 _μ_m for panels a, c and e; Bar=20 _μ_m for panels b, d, and f. CEPO, carbamylated rhEPO; EPO, erythropoietin; rhEPO, recombinant human erythropoietin; TUNEL, deoxynucleotidyl transferase-mediated biotinylated UTP nick end labelling model. *P<0.05 vs the vehicle group.
Comment in
- Neuroprotection with or without erythropoiesis; sometimes less is more.
Torup L. Torup L. Br J Pharmacol. 2007 Aug;151(8):1141-2. doi: 10.1038/sj.bjp.0707287. Epub 2007 May 29. Br J Pharmacol. 2007. PMID: 17533424 Free PMC article.
References
- Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–134. -PubMed
- Belayev L, Khoutorova L, Zhao W, Vigdorchik A, Belayev A, Busto R, et al. Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke. 2005;36:1071–1076. -PubMed
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. -PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS 42345/NS/NINDS NIH HHS/United States
- P01 NS023393/NS/NINDS NIH HHS/United States
- R01 NS 43324/NS/NINDS NIH HHS/United States
- R01 HL064766/HL/NHLBI NIH HHS/United States
- R01 NS043324/NS/NINDS NIH HHS/United States
- P01 NS042345/NS/NINDS NIH HHS/United States
- P01 NS 23393/NS/NINDS NIH HHS/United States
- R01 HL 64766/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical