Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages - PubMed (original) (raw)
Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages
Daniel E Voth et al. Infect Immun. 2007 Sep.
Abstract
Coxiella burnetii, the cause of human Q fever, is an aerosol-borne, obligate intracellular bacterium that targets host alveolar mononuclear phagocytic cells during infection. In all cell types examined, C. burnetii establishes a replicative niche in a lysosome-like parasitophorous vacuole where it carries out a lengthy infectious cycle with minimal cytopathic effects. The persistent and mild nature of C. burnetii infection in vitro suggests that the pathogen modulates apoptosis to sustain the host cell. In the current study, we examined the ability of C. burnetii to inhibit apoptotic cell death during infection of human THP-1 monocyte-derived macrophages and primary monkey alveolar macrophages. C. burnetii-infected cells demonstrated significant protection from death relative to uninfected cells following treatment with staurosporine, a potent inducer of intrinsic apoptosis. This protection correlated with reduced cleavage of caspase-9, caspase-3, and poly(ADP-ribose) polymerase (PARP), all proteolytic events that occur during apoptosis. Reduced PARP cleavage was also observed in cells treated with tumor necrosis factor alpha to induce extrinsic apoptosis. Apoptosis inhibition was a C. burnetii-driven process as infected cells treated with rifampin or chloramphenicol, inhibitors of bacterial RNA and protein synthesis, respectively, showed significantly reduced protection against staurosporine-induced apoptosis. C. burnetii infection affected the expression of multiple apoptosis-related genes and resulted in increased synthesis of the antiapoptotic proteins A1/Bfl-1 and c-IAP2. Collectively, these data suggest that C. burnetii modulates apoptotic pathways to inhibit host cell death, thus providing a stable, intracellular niche for the course of the pathogen's infectious cycle.
Figures
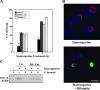
FIG. 1.
C. burnetii inhibits staurosporine-induced death of THP-1 cells. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. (A) Infected and uninfected cells were treated with staurosporine for 4 h and subsequently visualized by light microscopy. Treated uninfected cells demonstrated morphological changes consistent with apoptosis (e.g., membrane blebbing and cell shrinking) that were not observed with treated infected cells that maintained distinct PVs (arrows). Bar, 20 μm. (B) Infected and uninfected cells were incubated with staurosporine for 4 or 8 h. Cell viability was then assessed via WST-8 staining, and viability was calculated as described in Materials and Methods. Results are expressed as percent cell death compared to untreated cells. Experiments were conducted in triplicate, and error bars represent the standard deviation from the mean. An asterisk indicates a P value of <0.05 in comparison to uninfected cell cultures as determined by a Student's t test. Infected cells demonstrated over 50% less death than uninfected cells when treated with staurosporine.
FIG. 2.
C. burnetii inhibits apoptotic cell death of THP-1 cells. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. Infected and uninfected cells were then treated with staurosporine for 2 h to induce apoptosis. Untreated infected cells were used as a control. Cells were immunolabeled for confocal fluorescence microscopy by using antibodies directed against C. burnetii (red) and cleaved PARP (green). DRAQ5 was used to stain DNA (blue). Untreated and treated infected cell cultures contained significantly fewer cleaved PARP-positive (apoptotic) cells than treated uninfected cell cultures. Bar, 10 μm.
FIG. 3.
C. burnetii infection inhibits caspase and PARP processing in THP-1 cells and monkey primary alveolar macrophages. Uninfected cells or cells infected with the C. burnetii Nine Mile phase II strain for 48 h were treated with staurosporine for 2 or 4 h or with TNF-α for 8 h. Cells were lysed, and equal amounts of protein were subjected to immunoblot analysis. (A) Lysates of staurosporine-treated THP-1 cells probed for caspase-9, caspase-3, and PARP. Cleaved forms of all proteins were dramatically reduced in infected cell lysates. (B) Lysates of staurosporine-treated monkey primary alveolar macrophages probed for caspase-3 and PARP. Similar to THP-1 lysates, cleaved forms of caspase-3 and PARP were substantially reduced in infected cell lysates. (C) Lysates of TNF-α-treated THP-1 cells probed for PARP. Similar to staurosporine-treated cells, cleaved PARP was dramatically reduced in infected cell lysates.
FIG. 4.
C. burnetii protein synthesis is required for antiapoptotic activity. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h in the presence or absence of the antibiotics rifampin (Rif) or chloramphenicol (Cm) to inhibit bacterial RNA and protein synthesis, respectively. (A) Infected and uninfected cell cultures were treated with staurosporine for 4 or 8 h and assessed for viability using WST-8 staining. Experiments were conducted in triplicate, and error bars represent the standard deviation from the mean. An asterisk indicates a P value of <0.05 in comparison to uninfected cells and infected cells treated with antibiotics as determined by a Student's t test. Antibiotic-treated infected cell cultures demonstrated levels of death similar to those of uninfected cell cultures. (B) Cells infected in the presence or absence of rifampin were treated with staurosporine for 2 h. Cells were immunostained for confocal fluorescence microscopy by using antibodies directed against C. burnetii (red) and cleaved PARP (green). DNA (blue) was stained with DRAQ5. The percentage of cells staining positive for nuclear cleaved PARP following staurosporine treatment was significantly higher in infected cell cultures treated with rifampin. Bar, 10 μm. (C) Cells infected in the presence or absence of rifampin or chloramphenicol were treated with staurosporine for 2 h. Cell lysates were harvested, and equal amounts of protein were subjected to immunoblot analysis for cleaved PARP. Antibiotic treatment of infected cells resulted in increased levels of cleaved PARP following staurosporine treatment.
FIG. 5.
C. burnetii infection does not induce mass degradation of proapoptotic Bcl-2 family proteins. THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. Infected and uninfected cell cultures were then treated with staurosporine for 2 h. Cells were lysed, and equal amounts of protein were subjected to immunoblot analysis using Bad, Bak, Bax, Bik, Bim, or Bmf primary antibodies. Similar levels of these proteins were observed between infected and uninfected cells treated with staurosporine. The GAPDH immunoblot is included as a loading control.
FIG. 6.
C. burnetii infection modulates the expression of apoptosis-related genes. (A) Total RNA was extracted from uninfected THP-1 cells and cells infected with the C. burnetii Nine Mile phase II strain for 48 h. RT-PCR was performed using the RT2 Profiler Array. Genes showing an increase or decrease in expression greater than or equal to twofold relative to uninfected cells are shown. Depicted results are representative of two independent experiments. Twenty-nine genes were up- or down-regulated in response to C. burnetii infection, of which 12 indicated a proapoptotic response and 18 indicated an anti-apoptotic response. (B) THP-1 cells were infected with the C. burnetii Nine Mile phase II strain for 48 h. Infected and uninfected cell cultures were treated with staurosporine for 2 h as indicated. Cells were lysed, and equal amounts of protein were subjected to immunoblot analysis using A1/Bfl-1 or c-IAP2 primary antibodies. Consistent with gene expression data, increased levels of A1/Bfl-1 and c-IAP2 were observed in cell lysates of both untreated and staurosporine-treated infected cell cultures relative to lysates of uninfected cells.
Similar articles
- Coxiella burnetii induces apoptosis during early stage infection via a caspase-independent pathway in human monocytic THP-1 cells.
Zhang Y, Zhang G, Hendrix LR, Tesh VL, Samuel JE. Zhang Y, et al. PLoS One. 2012;7(1):e30841. doi: 10.1371/journal.pone.0030841. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303462 Free PMC article. - Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii antiapoptotic activity.
Voth DE, Heinzen RA. Voth DE, et al. Infect Immun. 2009 Jan;77(1):205-13. doi: 10.1128/IAI.01124-08. Epub 2008 Nov 3. Infect Immun. 2009. PMID: 18981248 Free PMC article. - Defying Death - How Coxiella burnetii Copes with Intentional Host Cell Suicide.
Cordsmeier A, Wagner N, Lührmann A, Berens C. Cordsmeier A, et al. Yale J Biol Med. 2019 Dec 20;92(4):619-628. eCollection 2019 Dec. Yale J Biol Med. 2019. PMID: 31866777 Free PMC article. Review. - Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria.
Lührmann A, Roy CR. Lührmann A, et al. Infect Immun. 2007 Nov;75(11):5282-9. doi: 10.1128/IAI.00863-07. Epub 2007 Aug 20. Infect Immun. 2007. PMID: 17709406 Free PMC article. - Coxiella burnetii as a useful tool to investigate bacteria-friendly host cell compartments.
Pechstein J, Schulze-Luehrmann J, Lührmann A. Pechstein J, et al. Int J Med Microbiol. 2018 Jan;308(1):77-83. doi: 10.1016/j.ijmm.2017.09.010. Epub 2017 Sep 14. Int J Med Microbiol. 2018. PMID: 28935173 Review.
Cited by
- Murine Alveolar Macrophages Are Highly Susceptible to Replication of Coxiella burnetii Phase II In Vitro.
Fernandes TD, Cunha LD, Ribeiro JM, Massis LM, Lima-Junior DS, Newton HJ, Zamboni DS. Fernandes TD, et al. Infect Immun. 2016 Aug 19;84(9):2439-48. doi: 10.1128/IAI.00411-16. Print 2016 Sep. Infect Immun. 2016. PMID: 27297388 Free PMC article. - Virulent Coxiella burnetii pathotypes productively infect primary human alveolar macrophages.
Graham JG, MacDonald LJ, Hussain SK, Sharma UM, Kurten RC, Voth DE. Graham JG, et al. Cell Microbiol. 2013 Jun;15(6):1012-25. doi: 10.1111/cmi.12096. Epub 2013 Jan 14. Cell Microbiol. 2013. PMID: 23279051 Free PMC article. - Host Kinase Activity is Required for Coxiella burnetii Parasitophorous Vacuole Formation.
Hussain SK, Broederdorf LJ, Sharma UM, Voth DE. Hussain SK, et al. Front Microbiol. 2010 Dec 23;1:137. doi: 10.3389/fmicb.2010.00137. eCollection 2010. Front Microbiol. 2010. PMID: 21772829 Free PMC article. - Undercover Agents of Infection: The Stealth Strategies of T4SS-Equipped Bacterial Pathogens.
Bienvenu A, Martinez E, Bonazzi M. Bienvenu A, et al. Toxins (Basel). 2021 Oct 9;13(10):713. doi: 10.3390/toxins13100713. Toxins (Basel). 2021. PMID: 34679006 Free PMC article. Review. - The anti-apoptotic Coxiella burnetii effector protein AnkG is a strain specific virulence factor.
Schäfer W, Schmidt T, Cordsmeier A, Borges V, Beare PA, Pechstein J, Schulze-Luehrmann J, Holzinger J, Wagner N, Berens C, Heydel C, Gomes JP, Lührmann A. Schäfer W, et al. Sci Rep. 2020 Sep 21;10(1):15396. doi: 10.1038/s41598-020-72340-9. Sci Rep. 2020. PMID: 32958854 Free PMC article.
References
- Abu-Zant, A., S. Jones, R. Asare, J. Suttles, C. Price, J. Graham, and Y. A. Kwaik. 2006. Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell. Microbiol. 9:246-264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous