Oral transmissibility of prion disease is enhanced by binding to soil particles - PubMed (original) (raw)
Oral transmissibility of prion disease is enhanced by binding to soil particles
Christopher J Johnson et al. PLoS Pathog. 2007 Jul.
Abstract
Soil may serve as an environmental reservoir for prion infectivity and contribute to the horizontal transmission of prion diseases (transmissible spongiform encephalopathies [TSEs]) of sheep, deer, and elk. TSE infectivity can persist in soil for years, and we previously demonstrated that the disease-associated form of the prion protein binds to soil particles and prions adsorbed to the common soil mineral montmorillonite (Mte) retain infectivity following intracerebral inoculation. Here, we assess the oral infectivity of Mte- and soil-bound prions. We establish that prions bound to Mte are orally bioavailable, and that, unexpectedly, binding to Mte significantly enhances disease penetrance and reduces the incubation period relative to unbound agent. Cox proportional hazards modeling revealed that across the doses of TSE agent tested, Mte increased the effective infectious titer by a factor of 680 relative to unbound agent. Oral exposure to Mte-associated prions led to TSE development in experimental animals even at doses too low to produce clinical symptoms in the absence of the mineral. We tested the oral infectivity of prions bound to three whole soils differing in texture, mineralogy, and organic carbon content and found soil-bound prions to be orally infectious. Two of the three soils increased oral transmission of disease, and the infectivity of agent bound to the third organic carbon-rich soil was equivalent to that of unbound agent. Enhanced transmissibility of soil-bound prions may explain the environmental spread of some TSEs despite the presumably low levels shed into the environment. Association of prions with inorganic microparticles represents a novel means by which their oral transmission is enhanced relative to unbound agent.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
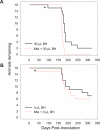
Figure 1. No Loss of Oral TSE Transmissibility Following Sorption of Prions from Infected BH to Mte (BH–Mte Mixtures)
The oral transmissibility of prions in 30 (A) and 3 (B) μL was not diminished by dosing with Mte. * indicates non-TSE intercurrent death. Animals dosed with Mte alone remained healthy throughout the course of the experiment (unpublished data).
Figure 2. Mte Enhances Oral TSE Transmission at a Low Dose of Infected BH (BH–Mte Mixtures)
Ingestion of Mte mixed with a lower dose of TSE-infected BH (0.3 μL) markedly shortens incubation period and increases disease penetrance relative to an equal amount of unbound BH. * indicates non-TSE intercurrent death. Animals dosed with Mte alone remained healthy throughout the course of the experiment (unpublished data).
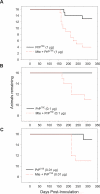
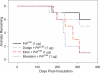
Figure 3. Concurrent Peroral Administration of Mte and PrPTSE Dramatically Increases Disease Penetrance at Agent Doses That Typically Fail to Produce Clinical Symptoms (PrPTSE–Mte Mixture)
(A) Mte increases disease penetrance and shortens incubation periods associated with ingestion of 1 μg of purified PrPTSE. Concurrent peroral dosage of lower, typically subclinical doses of purified PrPTSE (0.1 or 0.01 μg, [B and C]) with Mte increases disease incidence. Animals dosed with Mte alone remained healthy throughout the course of the experiment (unpublished data).
Figure 4. Maintenance of Strain Properties for Mte-Associated PrPTSE
BH from hamsters clinically affected with either HY or DY agents were incubated with Mte to allow binding. Desorbed proteins were analyzed by SDS-PAGE and immunoblotting. Cleavage patterns of PrPHY and PrPDY extracted from Mte parallel PK cleavage patterns for the respective proteins: cleaved PrPDY migrates further (corresponding to a 1- to 2-kDa molecular mass difference) than cleaved PrPHY. Immunoblot used the PrP-specific antibody 3F4.
Figure 5. Prions Bound to Whole Soils Remain Orally Infectious and Some Soils Increase Transmission
Three soils (Dodge, Elliot, and Bluestem) were incubated in the presence of purified PrPTSE. The samples were orally dosed into hamsters and found to remain orally infectious. Agent association with Elliot and Bluestem soils increases disease incidence, whereas Dodge soil does not influence disease transmission. Animals dosed with soil alone remained healthy throughout the course of the experiment (unpublished data).
Similar articles
- Prions adhere to soil minerals and remain infectious.
Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Johnson CJ, et al. PLoS Pathog. 2006 Apr;2(4):e32. doi: 10.1371/journal.ppat.0020032. Epub 2006 Apr 14. PLoS Pathog. 2006. PMID: 16617377 Free PMC article. - Estimating prion adsorption capacity of soil by BioAssay of Subtracted Infectivity from Complex Solutions (BASICS).
Wyckoff AC, Lockwood KL, Meyerett-Reid C, Michel BA, Bender H, VerCauteren KC, Zabel MD. Wyckoff AC, et al. PLoS One. 2013;8(3):e58630. doi: 10.1371/journal.pone.0058630. Epub 2013 Mar 4. PLoS One. 2013. PMID: 23484043 Free PMC article. - Fate of prions in soil: a review.
Smith CB, Booth CJ, Pedersen JA. Smith CB, et al. J Environ Qual. 2011 Mar-Apr;40(2):449-61. doi: 10.2134/jeq2010.0412. J Environ Qual. 2011. PMID: 21520752 Free PMC article. Review. - Direct detection of soil-bound prions.
Genovesi S, Leita L, Sequi P, Andrighetto I, Sorgato MC, Bertoli A. Genovesi S, et al. PLoS One. 2007 Oct 24;2(10):e1069. doi: 10.1371/journal.pone.0001069. PLoS One. 2007. PMID: 17957252 Free PMC article. - Insights into prion strains and neurotoxicity.
Aguzzi A, Heikenwalder M, Polymenidou M. Aguzzi A, et al. Nat Rev Mol Cell Biol. 2007 Jul;8(7):552-61. doi: 10.1038/nrm2204. Nat Rev Mol Cell Biol. 2007. PMID: 17585315 Review.
Cited by
- Characterisation of European Field Goat Prion Isolates in Ovine PrP Overexpressing Transgenic Mice (Tgshp IX) Reveals Distinct Prion Strains.
Ernst S, Nonno R, Langeveld J, Andreoletti O, Acin C, Papasavva-Stylianou P, Sklaviadis T, Acutis PL, van Keulen L, Spiropoulos J, Keller M, Groschup MH, Fast C. Ernst S, et al. Pathogens. 2024 Jul 27;13(8):629. doi: 10.3390/pathogens13080629. Pathogens. 2024. PMID: 39204230 Free PMC article. - Prion Seeding Activity in Plant Tissues Detected by RT-QuIC.
Burgener K, Lichtenberg SS, Walsh DP, Inzalaco HN, Lomax A, Pedersen JA. Burgener K, et al. Pathogens. 2024 May 26;13(6):452. doi: 10.3390/pathogens13060452. Pathogens. 2024. PMID: 38921750 Free PMC article. - Detection of Chronic Wasting Disease Prions in Prairie Soils from Endemic Regions.
Kuznetsova A, Ness A, Moffatt E, Bollinger T, McKenzie D, Stasiak I, Bahnson CS, Aiken JM. Kuznetsova A, et al. Environ Sci Technol. 2024 Jun 25;58(25):10932-10940. doi: 10.1021/acs.est.4c04633. Epub 2024 Jun 12. Environ Sci Technol. 2024. PMID: 38865602 Free PMC article. - Modeling the Impact of Climate Change on Cervid Chronic Wasting Disease in Semi-Arid South Texas.
Islam MR, Bulut U, Feria-Arroyo TP, Tyshenko MG, Oraby T. Islam MR, et al. Front Epidemiol. 2022 May 26;2:889280. doi: 10.3389/fepid.2022.889280. eCollection 2022. Front Epidemiol. 2022. PMID: 38455276 Free PMC article. - Chronic Wasting Disease: State of the Science.
Bartz JC, Benavente R, Caughey B, Christensen S, Herbst A, Hoover EA, Mathiason CK, McKenzie D, Morales R, Schwabenlander MD, Walsh DP, The Nc North American Interdisciplinary Chronic Wasting Disease Research Consortium Members. Bartz JC, et al. Pathogens. 2024 Feb 2;13(2):138. doi: 10.3390/pathogens13020138. Pathogens. 2024. PMID: 38392876 Free PMC article. Review.
References
- Zou WQ, Gambetti P. From microbes to prions: The final proof of the prion hypothesis. Cell. 2005;121:155–157. - PubMed
- Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev Sci Tech. 1996;15:827–852. - PubMed
- Greig JR. Scrapie: Observations on the transmission of the disease by mediate contact. Vet J. 1940;96:203–206.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources