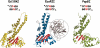Nucleotide flipping by restriction enzymes analyzed by 2-aminopurine steady-state fluorescence - PubMed (original) (raw)
Nucleotide flipping by restriction enzymes analyzed by 2-aminopurine steady-state fluorescence
Gintautas Tamulaitis et al. Nucleic Acids Res. 2007.
Abstract
Many DNA modification and repair enzymes require access to DNA bases and therefore flip nucleotides. Restriction endonucleases (REases) hydrolyze the phosphodiester backbone within or in the vicinity of the target recognition site and do not require base extrusion for the sequence readout and catalysis. Therefore, the observation of extrahelical nucleotides in a co-crystal of REase Ecl18kI with the cognate sequence, CCNGG, was unexpected. It turned out that Ecl18kI reads directly only the CCGG sequence and skips the unspecified N nucleotides, flipping them out from the helix. Sequence and structure conservation predict nucleotide flipping also for the complexes of PspGI and EcoRII with their target DNAs (/CCWGG), but data in solution are limited and indirect. Here, we demonstrate that Ecl18kI, the C-terminal domain of EcoRII (EcoRII-C) and PspGI enhance the fluorescence of 2-aminopurines (2-AP) placed at the centers of their recognition sequences. The fluorescence increase is largest for PspGI, intermediate for EcoRII-C and smallest for Ecl18kI, probably reflecting the differences in the hydrophobicity of the binding pockets within the protein. Omitting divalent metal cations and mutation of the binding pocket tryptophan to alanine strongly increase the 2-AP signal in the Ecl18kI-DNA complex. Together, our data provide the first direct evidence that Ecl18kI, EcoRII-C and PspGI flip nucleotides in solution.
Figures
Figure 1.
Flipped nucleotides in the Ecl18kI–DNA complex structure (2FQZ). (A) General view of the Ecl18kI dimer–DNA complex. Protein is shown in spacefill. Residues 60–69 and 91–136 are removed for clarity. DNA is depicted in red. (B) Binding ‘pocket’ for the flipped out base. A flipped adenine base is accommodated in the ‘pocket’ made by the side chain atoms of Arg57 on one face and the indole ring of Trp61 on the other face.
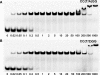
Figure 2.
Gel mobility shift analysis of the interactions between Ecl18kI and oligoduplexes. (A) Ecl18kI binding of the oligoduplex III containing the recognition sequence. (B) Ecl18kI binding of the cognate oligoduplex I containing 2-AP instead of the central A base. The binding reactions contained 33P-labeled 25 bp oligoduplex (0.1 nM) and the Ecl18kI at concentrations as indicated by each lane. Samples were analyzed by PAGE under non-denaturing conditions (see, Material and Methods Section). Gels were run in the presence of 5 mM of Ca2+ ions.
Figure 3.
Fluorescence study of base flipping by Ecl18kI in solution. Titration of 250 nM 2-AP containing oligoduplex I with increasing amounts of Ecl18kI in the presence (A) and absence (D) of Ca2+ ions, respectively. Corrected 2-AP emission spectra of Ecl18kI–DNA complexes (1250 nM Ecl18kI and 250 nM oligoduplexes I or II) in the presence (B) and absence (E) of Ca2+ ions (see Materials and Methods Section for the details). Diagrams in (C) and (F) illustrate the maximum fluorescence intensity values of the corrected fluorescence emission spectra presented in (B) and (E), respectively.
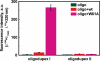
Figure 4.
2-AP fluorescence in the ternary complexes of the wt Ecl18kI and W61A mutant. Diagrams illustrate the fluorescence intensity values of the corrected fluorescence emission spectra at fluorescence maximum (367 nm for wt, 365 nm for W61A and 370 nm for oligoduplexes). Reactions contained 1250 nM protein and 250 nM oligoduplexes I or II and were measured in the presence of Ca2+ ions.
Figure 5.
Ecl18kI, EcoRII and PspGI monomer structures. Central β-sheets are colored in red. N-terminal effector domain which is unique to EcoRII (44), is shown in gray. PspGI structural model (26) is shown.
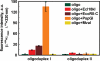
Figure 6.
2-AP fluorescence in the ternary complexes of EcoRII-C, PspGI and MvaI. Diagrams show the fluorescence intensity values of the corrected fluorescence emission spectra at fluorescence maximum (360 nm for EcoRII-C and PspGI, 370 nm for MvaI and oligoduplexes). Reactions contained 1250 nM protein and 250 nM oligoduplexes I or II and were measured in the presence of Ca2+ ions.
Similar articles
- How PspGI, catalytic domain of EcoRII and Ecl18kI acquire specificities for different DNA targets.
Tamulaitis G, Zaremba M, Szczepanowski RH, Bochtler M, Siksnys V. Tamulaitis G, et al. Nucleic Acids Res. 2008 Nov;36(19):6101-8. doi: 10.1093/nar/gkn621. Epub 2008 Sep 27. Nucleic Acids Res. 2008. PMID: 18820295 Free PMC article. - Time-resolved fluorescence studies of nucleotide flipping by restriction enzymes.
Neely RK, Tamulaitis G, Chen K, Kubala M, Siksnys V, Jones AC. Neely RK, et al. Nucleic Acids Res. 2009 Nov;37(20):6859-70. doi: 10.1093/nar/gkp688. Epub 2009 Sep 8. Nucleic Acids Res. 2009. PMID: 19740769 Free PMC article. - Central base pair flipping and discrimination by PspGI.
Szczepanowski RH, Carpenter MA, Czapinska H, Zaremba M, Tamulaitis G, Siksnys V, Bhagwat AS, Bochtler M. Szczepanowski RH, et al. Nucleic Acids Res. 2008 Nov;36(19):6109-17. doi: 10.1093/nar/gkn622. Epub 2008 Oct 1. Nucleic Acids Res. 2008. PMID: 18829716 Free PMC article. - Fluorescent DNA base modifications and substitutes: multiple fluorophore labeling and the DETEQ concept.
Wagenknecht HA. Wagenknecht HA. Ann N Y Acad Sci. 2008;1130:122-30. doi: 10.1196/annals.1430.001. Epub 2007 Dec 20. Ann N Y Acad Sci. 2008. PMID: 18096856 Review. - Artificial restriction DNA cutters as new tools for gene manipulation.
Katada H, Komiyama M. Katada H, et al. Chembiochem. 2009 May 25;10(8):1279-88. doi: 10.1002/cbic.200900040. Chembiochem. 2009. PMID: 19396851 Review.
Cited by
- Unique mechanism of target recognition by PfoI restriction endonuclease of the CCGG-family.
Tamulaitiene G, Manakova E, Jovaisaite V, Tamulaitis G, Grazulis S, Bochtler M, Siksnys V. Tamulaitiene G, et al. Nucleic Acids Res. 2019 Jan 25;47(2):997-1010. doi: 10.1093/nar/gky1137. Nucleic Acids Res. 2019. PMID: 30445642 Free PMC article. - Chemical mapping of cytosines enzymatically flipped out of the DNA helix.
Daujotyte D, Liutkeviciūte Z, Tamulaitis G, Klimasauskas S. Daujotyte D, et al. Nucleic Acids Res. 2008 Jun;36(10):e57. doi: 10.1093/nar/gkn200. Epub 2008 May 1. Nucleic Acids Res. 2008. PMID: 18450817 Free PMC article. - Restriction endonuclease TseI cleaves A:A and T:T mismatches in CAG and CTG repeats.
Ma L, Chen K, Clarke DJ, Nortcliffe CP, Wilson GG, Edwardson JM, Morton AJ, Jones AC, Dryden DT. Ma L, et al. Nucleic Acids Res. 2013 May;41(9):4999-5009. doi: 10.1093/nar/gkt176. Epub 2013 Mar 23. Nucleic Acids Res. 2013. PMID: 23525471 Free PMC article. - Functional significance of protein assemblies predicted by the crystal structure of the restriction endonuclease BsaWI.
Tamulaitis G, Rutkauskas M, Zaremba M, Grazulis S, Tamulaitiene G, Siksnys V. Tamulaitis G, et al. Nucleic Acids Res. 2015 Sep 18;43(16):8100-10. doi: 10.1093/nar/gkv768. Epub 2015 Aug 3. Nucleic Acids Res. 2015. PMID: 26240380 Free PMC article. - Base flipping in tn10 transposition: an active flip and capture mechanism.
Bischerour J, Chalmers R. Bischerour J, et al. PLoS One. 2009 Jul 10;4(7):e6201. doi: 10.1371/journal.pone.0006201. PLoS One. 2009. PMID: 19593448 Free PMC article.
References
- Klimasauskas S, Kumar S, Roberts RJ, Cheng X. HhaI methyltransferase flips its target base out of the DNA helix. Cell. 1994;76:357–369. - PubMed
- Reinisch KM, Chen L, Verdine GL, Lipscomb WN. The crystal structure of HaeIII methyltransferase convalently complexed to DNA: an extrahelical cytosine and rearranged base pairing. Cell. 1995;82:143–153. - PubMed
- Goedecke K, Pignot M, Goody RS, Scheidig AJ, Weinhold E. Structure of the N6-adenine DNA methyltransferase M.TaqI in complex with DNA and a cofactor analog. Nat. Struct. Biol. 2001;8:121–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases