Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2007 Sep;51(9):3304-10.
doi: 10.1128/AAC.01318-06. Epub 2007 Jul 9.
Affiliations
- PMID: 17620371
- PMCID: PMC2043189
- DOI: 10.1128/AAC.01318-06
Randomized Controlled Trial
Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial
Samir G Sakka et al. Antimicrob Agents Chemother. 2007 Sep.
Abstract
Beta-lactams are regularly administered in intermittent short-term infusions. The percentage of the dosing interval during which free drug concentrations exceed the MIC (fT(>MIC)) is the measure of drug exposure that best correlates with clinical outcome for beta-lactams. Therefore, administration by continuous infusion has gained increasing interest recently. We studied 20 critically ill patients with nosocomial pneumonia and investigated whether continuous infusion with a reduced total dose, compared to the standard regimen of intermittent short-term infusion, results in a superior probability of target attainment as assessed by the fT(>MIC) value of imipenem. In this prospective, randomized, controlled clinical study, patients received either a loading dose of 1 g/1 g imipenem and cilastatin (as a short-term infusion) at time zero, followed by 2 g/2 g imipenem-cilastatin per 24 h as a continuous infusion for 3 days (n = 10), or 1 g/1 g imipenem-cilastatin three times per day as a short-term infusion for 3 days (total daily dose, 3 g/3 g; n = 10). Imipenem concentrations in plasma were determined by using a validated liquid chromatography-tandem mass spectrometry assay. A two-compartment open model was employed for population pharmacokinetic modeling. We simulated 10,000 intensive-care-unit patients via Monte Carlo simulations for pharmacodynamic evaluation using the target 40% fT(>MIC). The probability of target attainment by MIC for intermittent infusion was robust (>90%) up to MICs of 1 to 2 mg/liter. The corresponding value for continuous infusion was 2 to 4 mg/liter. Although all 20 patients had an fT(>MIC) of 100%, 3 patients died. Patient survival was best described by employing a sepsis-related organ failure assessment score as a covariate in a logistic regression analysis. Larger clinical trials are warranted for evaluation of continuous infusions at a reduced dose of imipenem for critically ill patients.
Figures
FIG. 1.
Observed concentrations in plasma (average ± SD) and typical predicted concentration-time profiles for imipenem after continuous and intermittent treatment of critically ill patients with normal renal function (see Materials and Methods for details on dosage regimens). The typical concentration-time profile based on the median pharmacokinetic parameters was predicted for the continuous-infusion group (continuous line) and for the intermittent-treatment group (dashed line). Importantly, this line is not equivalent to the average predicted concentrations for a large number of simulated patients. The two lines fall on top of each other during the first 4 h.
FIG. 2.
Observed-predicted plot for imipenem for all modes of administration. The line of best fit was determined as follows: observed = 1.023 × predicted + 0.262; _r_2 = 0.919; P ≪ 0.001.
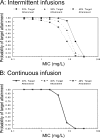
FIG. 3.
Probability of target attainment for imipenem administered as intermittent 40-min infusions at a dose of 1 g q8h (3 g/day) (A) or as continuous infusion at a dose of 2 g/day (B). We studied the _fT_>MIC targets of 20%, 30%, and 40% of the dosing interval.
Similar articles
- Pharmacokinetics of imipenem in critically ill patients during empirical treatment of nosocomial pneumonia: a comparison of 0.5-h and 3-h infusions.
Lipš M, Siller M, Strojil J, Urbánek K, Balík M, Suchánková H. Lipš M, et al. Int J Antimicrob Agents. 2014 Oct;44(4):358-62. doi: 10.1016/j.ijantimicag.2014.05.011. Epub 2014 Jun 25. Int J Antimicrob Agents. 2014. PMID: 25216543 Clinical Trial. - Comparison of 30-min and 3-h infusion regimens for imipenem/cilastatin and for meropenem evaluated by Monte Carlo simulation.
Lee LS, Kinzig-Schippers M, Nafziger AN, Ma L, Sörgel F, Jones RN, Drusano GL, Bertino JS Jr. Lee LS, et al. Diagn Microbiol Infect Dis. 2010 Nov;68(3):251-8. doi: 10.1016/j.diagmicrobio.2010.06.012. Epub 2010 Sep 18. Diagn Microbiol Infect Dis. 2010. PMID: 20851549 Clinical Trial. - Is continuous infusion of imipenem always the best choice?
Suchánková H, Lipš M, Urbánek K, Neely MN, Strojil J. Suchánková H, et al. Int J Antimicrob Agents. 2017 Mar;49(3):348-354. doi: 10.1016/j.ijantimicag.2016.12.005. Epub 2017 Feb 9. Int J Antimicrob Agents. 2017. PMID: 28189734 - Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.
Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, Idowu T, Domalaon R, Schweizer F, Zhanel MA, Lagacé-Wiens PRS, Walkty AJ, Noreddin A, Lynch Iii JP, Karlowsky JA. Zhanel GG, et al. Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review. - Prolonged administration of β-lactam antibiotics - a comprehensive review and critical appraisal.
Osthoff M, Siegemund M, Balestra G, Abdul-Aziz MH, Roberts JA. Osthoff M, et al. Swiss Med Wkly. 2016 Oct 10;146:w14368. doi: 10.4414/smw.2016.14368. eCollection 2016. Swiss Med Wkly. 2016. PMID: 27731492 Review.
Cited by
- Prolonged versus Intermittent Infusion of β-Lactams for the Treatment of Nosocomial Pneumonia: A Meta-Analysis.
Lal A, Jaoude P, El-Solh AA. Lal A, et al. Infect Chemother. 2016 Jun;48(2):81-90. doi: 10.3947/ic.2016.48.2.81. Epub 2016 Jun 30. Infect Chemother. 2016. PMID: 27433378 Free PMC article. - Parametric and Nonparametric Population Pharmacokinetic Models to Assess Probability of Target Attainment of Imipenem Concentrations in Critically Ill Patients.
de Velde F, de Winter BCM, Neely MN, Strojil J, Yamada WM, Harbarth S, Huttner A, van Gelder T, Koch BCP, Muller AE, On Behalf Of The Combacte-Net Consortium. de Velde F, et al. Pharmaceutics. 2021 Dec 16;13(12):2170. doi: 10.3390/pharmaceutics13122170. Pharmaceutics. 2021. PMID: 34959451 Free PMC article. - Pharmacokinetic/pharmacodynamic profiling of imipenem in patients admitted to an intensive care unit in India: A nonrandomized, cross-sectional, analytical, open-labeled study.
Abhilash B, Tripathi CD, Gogia AR, Meshram GG, Kumar M, Suraj B. Abhilash B, et al. Indian J Crit Care Med. 2015 Oct;19(10):587-92. doi: 10.4103/0972-5229.167036. Indian J Crit Care Med. 2015. PMID: 26628823 Free PMC article. - Continuous versus intermittent administration of piperacillin-tazobactam in intensive care unit patients with ventilator-associated pneumonia.
Fahimi F, Ghafari S, Jamaati H, Baniasadi S, Tabarsi P, Najafi A, Akhzarmehr A, Hashemian SM. Fahimi F, et al. Indian J Crit Care Med. 2012 Jul;16(3):141-7. doi: 10.4103/0972-5229.102083. Indian J Crit Care Med. 2012. PMID: 23188954 Free PMC article. - Population pharmacokinetics of polymyxin B: a systematic review.
Chen N, Guo J, Xie J, Xu M, Hao X, Ma K, Rao Y. Chen N, et al. Ann Transl Med. 2022 Feb;10(4):231. doi: 10.21037/atm-22-236. Ann Transl Med. 2022. PMID: 35280373 Free PMC article. Review.
References
- Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. - PubMed
- Boucher, B. A., W. L. Hickerson, D. A. Kuhl, A. M. Bombassaro, and G. S. Jaresko. 1990. Imipenem pharmacokinetics in patients with burns. Clin. Pharmacol. Ther. 48:130-137. - PubMed
- Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. - PubMed
- Craig, W. A., S. Ebert, and Y. Watanabe. 1993. Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr 86. American Society for Microbiology, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical