Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells - PubMed (original) (raw)
Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells
Martin Leeb et al. J Cell Biol. 2007.
Abstract
The Polycomb group (PcG) gene Ring1B has been implicated in the repression of developmental control genes and X inactivation and is essential for embryogenesis. Ring1B protein contains a RING finger domain and functions as an E3 ubiquitin ligase that is crucial for the monoubiquitination of histone H2A (H2AK119ub1). Here, we study the function of Ring1B in mouse embryonic stem (ES) cells. The deletion of Ring1B causes the loss of several PcG proteins, showing an unanticipated function in the regulation of PcG protein levels. Derepression of lineage genes and an aberrant differentiation potential is observed in Ring1B-deficient ES cells. Despite a crucial function of Ring1B in establishing the chromosome-wide ubiquitination of histone H2A lysine 119 (H2AK119ub1) upon Xist expression in ES cells, the initiation of silencing by Xist is independent of Ring1B. Other chromatin marks associated with the initiation of X inactivation are not affected in Ring1B-deficient cells, suggesting compensation for the loss of Ring1B in X inactivation in contrast to the repression of lineage genes.
Figures
Figure 1.
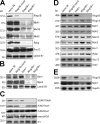
Generation of _Ring1B_-deficient ES cells. (A) Schematic representation of the Ring1B locus and the minus targeting vector replacing the start codon and the RING finger domain with a stop cassette. (B) Ring1B conditional targeting vector allowing for deletion of the Ring1B locus after Cre-mediated excision. (C–E) Southern analyses of 36_Ring1B_−/+ (C), 36_Ring1B_−_/cond_ (D), and 36_Ring1B_−_/_− (E) ES cells. Lanes were grouped where necessary. The white line indicates that intervening lanes have been spliced out. wt, wild type.
Figure 2.
Analysis of PcG expression in clone 36_Ring1B_−_/_− cells. (A) Western analysis of PcG proteins in nuclear extracts from control clone 36, 36_Ring1B_−_/cond_, and 36_Ring1B_−_/− ES cells. A representative lamin B1 loading control is shown. (B) Western analysis of Bmi1 and Mpc2 in nuclear extracts of clone 36, 36_Ring1B_−/cond_, 36_Eed_−_/−, and 36_Ring1B_−/− ES cells. (C) Western analysis of global levels of histone modifications associated with the initiation of X inactivation in clone 36, 36_Ring1B_−/cond_, 36_Eed_−_/−, and 36_Ring1B_−/_− ES cells. Ponceau-stained histone H1 bands show loading. (D) Expression analysis of PcG trancription by semiquantitative RT-PCR. (E) Northern analysis of Ring1B and Mph1 expression in ES cells. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as a loading control.
Figure 3.
Deregulation of developmental control genes upon the loss of Ring1B. (A) Expression analysis of Cdx2, Eomes, Pl-1, Hand1, Foxa2, Hnf4, Oct4, Hoxa1, Nestin, and the loading control β_-actin_ using RNA from ES cells as indicated by RT-PCR. (B and C) EBs derived from clone 36 and 36_Ring1B_−_/− ES cells after 3 wk of suspension culture. Images were obtained at 20× magnification. (D) Northern analysis of Pl-1 and Mph1 expression in clone 36 and 36_Ring1B_−/_− EBs. Bars, 1 mm.
Figure 4.
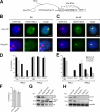
Recruitment of histone modifications and PcG proteins by Xist in _Ring1B_-deficient cells. (A) Scheme showing the inducible Xist expression system in clone 36 ES cells. In the presence of the inducer doxycycline, the tetracycline-regulated transactivator (nls-rtTA) binds to the inducible promoter (tetOP) and activates Xist, which then causes the repression of a puromycine selection marker gene (puro). (B and C) H2AK119ub1 immunofluorescence analysis combined with Xist RNA FISH of clone 36 and 36_Ring1B_−_/− ES (B) and differentiated cells (C). Bars, 5 μm. (D) Statistical analysis of the recruitment of PcG proteins and histone modifications by Xist in clone 36 and 36_Ring1B_−/− ES cells. Error bars represent SD (n > 100). Results for Ring1B, Mph1, and H2AK119ub1 in 36Ring1B−/knockin were counted once (n > 100). (E) Analysis of PcG protein recruitment and histone modifications by Xist in differentiated clone 36 and 36_Ring1B_−/− cells as in D. (F) Percentage of nuclei showing focal H4K20me1 staining in undifferentiated clone 36 and 36_Ring1B_−/− ES cells (n > 100). (G) Western analysis of global H3K27me3 and H2AK119ub1 in ES cells differentiated for 8 d as indicated. (H) Western analysis of the PRC2 proteins Suz12 and Ezh2 in nuclear extracts from clone 36, 36_Eed_−/−, and 36_Ring1B_−/_− cells that were differentiated for 8 d. Lamin B1 was used as a loading control.
Figure 5.
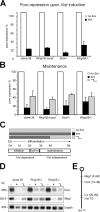
Initiation and maintenance of _Xist_-mediated silencing in _Ring1B_-deficient cells. (A) Quantification of puro repression upon Xist induction with doxycycline (dox) in clone 36, 36_Ring1B_−_/cond_, 36_Eed_−_/−, and 36_Ring1B_−/_− ES cells by Northern analysis. (B) Maintenance of puro repression in differentiated ES cells of indicated genotypes quantified by Northern analysis. Error bars represent SD. (C) Schematic representation of the doxycycline induction scheme (light gray, no dox; dark gray, +dox) used for the experiment in B. The phases of X inactivation are indicated below. (D) Stable maintenance of chromosome-wide silencing in the absence of Xist expression as shown for Meg1 and Puro by Northern analysis. (E) Scheme showing the location of Meg1, Cct4, and the Xist transgene on chromosome 11.
Similar articles
- Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells.
van der Stoop P, Boutsma EA, Hulsman D, Noback S, Heimerikx M, Kerkhoven RM, Voncken JW, Wessels LF, van Lohuizen M. van der Stoop P, et al. PLoS One. 2008 May 21;3(5):e2235. doi: 10.1371/journal.pone.0002235. PLoS One. 2008. PMID: 18493325 Free PMC article. - Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing.
Schoeftner S, Sengupta AK, Kubicek S, Mechtler K, Spahn L, Koseki H, Jenuwein T, Wutz A. Schoeftner S, et al. EMBO J. 2006 Jul 12;25(13):3110-22. doi: 10.1038/sj.emboj.7601187. Epub 2006 Jun 8. EMBO J. 2006. PMID: 16763550 Free PMC article. - Maintenance of undifferentiated state and self-renewal of embryonic neural stem cells by Polycomb protein Ring1B.
Román-Trufero M, Méndez-Gómez HR, Pérez C, Hijikata A, Fujimura Y, Endo T, Koseki H, Vicario-Abejón C, Vidal M. Román-Trufero M, et al. Stem Cells. 2009 Jul;27(7):1559-70. doi: 10.1002/stem.82. Stem Cells. 2009. PMID: 19544461 - Role of polycomb proteins Ring1A and Ring1B in the epigenetic regulation of gene expression.
Vidal M. Vidal M. Int J Dev Biol. 2009;53(2-3):355-70. doi: 10.1387/ijdb.082690mv. Int J Dev Biol. 2009. PMID: 19412891 Review. - Writing Histone Monoubiquitination in Human Malignancy-The Role of RING Finger E3 Ubiquitin Ligases.
Marsh DJ, Dickson KA. Marsh DJ, et al. Genes (Basel). 2019 Jan 18;10(1):67. doi: 10.3390/genes10010067. Genes (Basel). 2019. PMID: 30669413 Free PMC article. Review.
Cited by
- CBX8, a polycomb group protein, is essential for MLL-AF9-induced leukemogenesis.
Tan J, Jones M, Koseki H, Nakayama M, Muntean AG, Maillard I, Hess JL. Tan J, et al. Cancer Cell. 2011 Nov 15;20(5):563-75. doi: 10.1016/j.ccr.2011.09.008. Cancer Cell. 2011. PMID: 22094252 Free PMC article. - Epigenetic regulation of pluripotency and differentiation.
Boland MJ, Nazor KL, Loring JF. Boland MJ, et al. Circ Res. 2014 Jul 7;115(2):311-24. doi: 10.1161/CIRCRESAHA.115.301517. Circ Res. 2014. PMID: 24989490 Free PMC article. Review. - Polycomb complexes in X chromosome inactivation.
Brockdorff N. Brockdorff N. Philos Trans R Soc Lond B Biol Sci. 2017 Nov 5;372(1733):20170021. doi: 10.1098/rstb.2017.0021. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28947664 Free PMC article. Review. - Tissue-wide genetic and cellular landscape shapes the execution of sequential PRC2 functions in neural stem cell lineage progression.
Amberg N, Pauler FM, Streicher C, Hippenmeyer S. Amberg N, et al. Sci Adv. 2022 Nov 4;8(44):eabq1263. doi: 10.1126/sciadv.abq1263. Epub 2022 Nov 2. Sci Adv. 2022. PMID: 36322669 Free PMC article. - Genome-wide remodeling of the epigenetic landscape during myogenic differentiation.
Asp P, Blum R, Vethantham V, Parisi F, Micsinai M, Cheng J, Bowman C, Kluger Y, Dynlacht BD. Asp P, et al. Proc Natl Acad Sci U S A. 2011 May 31;108(22):E149-58. doi: 10.1073/pnas.1102223108. Epub 2011 May 5. Proc Natl Acad Sci U S A. 2011. PMID: 21551099 Free PMC article.
References
- Arrigoni, R., S.L. Alam, J.A. Wamstad, V.J. Bardwell, W.I. Sundquist, and N. Schreiber-Agus. 2006. The Polycomb-associated protein Rybp is a ubiquitin binding protein. FEBS Lett. 580:6233–6241. - PubMed
- Atsuta, T., S. Fujimura, H. Moriya, M. Vidal, T. Akasaka, and H. Koseki. 2001. Production of monoclonal antibodies against mammalian Ring1B proteins. Hybridoma. 20:43–46. - PubMed
- Ben-Saadon, R., D. Zaaroor, T. Ziv, and A. Ciechanover. 2006. The Polycomb protein Ring1B generates self atypical mixed ubiquitin chains required for its in vitro histone H2A ligase activity. Mol. Cell. 24:701–711. - PubMed
- Boyer, L.A., K. Plath, J. Zeitlinger, T. Brambrink, L.A. Medeiros, T.I. Lee, S.S. Levine, M. Wernig, A. Tajonar, M.K. Ray, et al. 2006. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 441:349–353. - PubMed