Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg - PubMed (original) (raw)
Comparative Study
Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg
Anouk C Vedder et al. PLoS One. 2007.
Abstract
Background: Two different enzyme preparations, agalsidase alfa (Replagal(TM), Shire) and beta (Fabrazyme(TM), Genzyme), are registered for treatment of Fabry disease. We compared the efficacy of and tolerability towards the two agalsidase preparations administered at identical protein dose in a randomized controlled open label trial.
Methodology/principal findings: Thirty-four Fabry disease patients were treated with either agalsidase alfa or agalsidase beta at equal dose of 0.2 mg/kg biweekly. Primary endpoint was reduction in left ventricular mass after 12 and 24 months of treatment. Other endpoints included occurrence of treatment failure (defined as progression of cardiac, renal or cerebral disease), glomerular filtration rate, pain, anti-agalsidase antibodies, and globotriaosylceramide levels in plasma and urine. After 12 and 24 months of treatment no reduction in left ventricular mass was seen, which was not different between the two treatment groups. Also, no differences in glomerular filtration rate, pain and decline in globotriaosylceramide levels were found. Antibodies developed only in males (4/8 in the agalsidase alfa group and 6/8 in the agalsidase beta group). Treatment failure within 24 months of therapy was seen in 8/34 patients: 6 male patients (3 in each treatment group) and 2 female patients (both agalsidase alfa). The occurrence of treatment failures did not differ between the two treatment groups; chi(2) = 0.38 p = 0.54.
Conclusion: Our study revealed no difference in reduction of left ventricular mass or other disease parameters after 12 and 24 months of treatment with either agalsidase alfa or beta at a dose of 0.2 mg/kg biweekly. Treatment failure occurred frequently in both groups and seems related to age and severe pre-treatment disease.
Trial registration: International Standard Randomized Clinical Trial ISRCTN45178534 [http://www.controlled-trials.com/ISRCTN45178534\].
Conflict of interest statement
Competing Interests: Author CH received reimbursement of expenses and small honoraria for lectures on the management of lysosomal storage diseases, including Fabry disease, from Genzyme Corporation, Shire and Actelion. All honoraria are donated to the Gaucher Stichting, a national foundation that supports research in the field of lysosomal storage disorders.
Figures
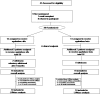
Figure 1. Study flow chart.
Δ clinical endpoint: change of primary endpoint from improvement of renal function to reduction in left ventricular mass.
Figure 2
A: Kaplan Meier survival analysis of percent patients with no treatment failure in both treatment groups. B: Number of patients with treatment failure displayed per 10 MSSI points. C: Number of patients with treatment failure displayed per age decade.
Similar articles
- Agalsidase alfa: a review of its use in the management of Fabry disease.
Keating GM. Keating GM. BioDrugs. 2012 Oct 1;26(5):335-54. doi: 10.2165/11209690-000000000-00000. BioDrugs. 2012. PMID: 22946754 Review. - Changes in plasma and urine globotriaosylceramide levels do not predict Fabry disease progression over 1 year of agalsidase alfa.
Schiffmann R, Ries M, Blankenship D, Nicholls K, Mehta A, Clarke JT, Steiner RD, Beck M, Barshop BA, Rhead W, West M, Martin R, Amato D, Nair N, Huertas P. Schiffmann R, et al. Genet Med. 2013 Dec;15(12):983-9. doi: 10.1038/gim.2013.56. Epub 2013 May 16. Genet Med. 2013. PMID: 23680766 Clinical Trial. - Agalsidase alfa in pediatric patients with Fabry disease: a 6.5-year open-label follow-up study.
Schiffmann R, Pastores GM, Lien YH, Castaneda V, Chang P, Martin R, Wijatyk A. Schiffmann R, et al. Orphanet J Rare Dis. 2014 Nov 26;9:169. doi: 10.1186/s13023-014-0169-6. Orphanet J Rare Dis. 2014. PMID: 25425121 Free PMC article. Clinical Trial. - Evaluation of the efficacy and safety of three dosing regimens of agalsidase alfa enzyme replacement therapy in adults with Fabry disease.
Goláň L, Goker-Alpan O, Holida M, Kantola I, Klopotowski M, Kuusisto J, Linhart A, Musial J, Nicholls K, Gonzalez-Rodriguez D, Sharma R, Vujkovac B, Chang P, Wijatyk A. Goláň L, et al. Drug Des Devel Ther. 2015 Jul 8;9:3435-44. doi: 10.2147/DDDT.S80928. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26185417 Free PMC article. Clinical Trial. - Enzyme replacement therapy for Anderson-Fabry disease.
El Dib R, Gomaa H, Carvalho RP, Camargo SE, Bazan R, Barretti P, Barreto FC. El Dib R, et al. Cochrane Database Syst Rev. 2016 Jul 25;7(7):CD006663. doi: 10.1002/14651858.CD006663.pub4. Cochrane Database Syst Rev. 2016. PMID: 27454104 Free PMC article. Review.
Cited by
- Mechanisms of Neutralizing Anti-drug Antibody Formation and Clinical Relevance on Therapeutic Efficacy of Enzyme Replacement Therapies in Fabry Disease.
Lenders M, Brand E. Lenders M, et al. Drugs. 2021 Nov;81(17):1969-1981. doi: 10.1007/s40265-021-01621-y. Epub 2021 Nov 8. Drugs. 2021. PMID: 34748189 Free PMC article. - Fabry nephropathy: 5 years of enzyme replacement therapy-a short review.
Barbey F, Lidove O, Schwarting A. Barbey F, et al. NDT Plus. 2008 Feb;1(1):11-19. doi: 10.1093/ndtplus/sfm022. Epub 2007 Dec 19. NDT Plus. 2008. PMID: 30792776 Free PMC article. No abstract available. - Hearing loss in adult patients with Fabry disease treated with enzyme replacement therapy.
Suntjens EB, Smid BE, Biegstraaten M, Dreschler WA, Hollak CE, Linthorst GE. Suntjens EB, et al. J Inherit Metab Dis. 2015 Mar;38(2):351-8. doi: 10.1007/s10545-014-9783-7. Epub 2014 Nov 14. J Inherit Metab Dis. 2015. PMID: 25395255 - Updated Evaluation of Agalsidase Alfa Enzyme Replacement Therapy for Patients with Fabry Disease: Insights from Real-World Data.
Feriozzi S, Chimenti C, Reisin RC. Feriozzi S, et al. Drug Des Devel Ther. 2024 Apr 3;18:1083-1101. doi: 10.2147/DDDT.S365885. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38585254 Free PMC article. Review. - Efficacy of Enzyme and Substrate Reduction Therapy with a Novel Antagonist of Glucosylceramide Synthase for Fabry Disease.
Ashe KM, Budman E, Bangari DS, Siegel CS, Nietupski JB, Wang B, Desnick RJ, Scheule RK, Leonard JP, Cheng SH, Marshall J. Ashe KM, et al. Mol Med. 2015 Apr 30;21(1):389-99. doi: 10.2119/molmed.2015.00088. Mol Med. 2015. PMID: 25938659 Free PMC article.
References
- Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, et al. Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. - PubMed
- Kint JA. Fabry's disease: alpha-galactosidase deficiency. Science. 1970;167:1268–1269. - PubMed
- Desnick RJ, Ioannou YA, Eng ME. α-Galactosidase A Deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease 8th ed Vol 3. New York: McGraw-Hill; 2001. pp. 3733–3774.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical