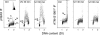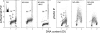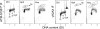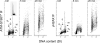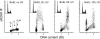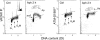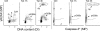Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents - PubMed (original) (raw)
Review
Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents
Toshiki Tanaka et al. Cytometry A. 2007 Sep.
Abstract
This review covers the topic of cytometric assessment of activation of Ataxia telangiectasia mutated (ATM) protein kinase and histone H2AX phosphorylation on Ser139 in response to DNA damage, particularly the damage that involves formation of DNA double-strand breaks. Briefly described are molecular mechanisms associated with activation of ATM and the downstream events that lead to recruitment of DNA repair machinery, engagement of cell cycle checkpoints, and activation of apoptotic pathway. Examples of multiparameter analysis of ATM activation and H2AX phosphorylation vis-a-vis cell cycle phase position and induction of apoptosis that employ flow- and laser scanning-cytometry are provided. They include cells treated with a variety of exogenous genotoxic agents, such as ionizing and UV radiation, DNA topoisomerase I (topotecan) and II (mitoxantrone, etoposide) inhibitors, nitric oxide-releasing aspirin, DNA replication inhibitors (aphidicolin, hydroxyurea, thymidine), and complex environmental carcinogens such as present in tobacco smoke. Also presented is an approach to identify DNA replicating (BrdU incorporating) cells based on selective photolysis of DNA that triggers H2AX phosphorylation. Listed are strategies to distinguish ATM activation and H2AX phosphorylation induced by primary DNA damage by genotoxic agents from those effects triggered by DNA fragmentation that takes place during apoptosis. While we review most published data, recent new findings also are included. Examples of multivariate analysis of ATM activation and H2AX phosphorylation presented in this review illustrate the advantages of cytometric flow- and image-analysis of these events in terms of offering a sensitive and valuable tool in studies of factors that induce DNA damage and/or affect DNA repair and allow one to explore the linkage between DNA damage, cell cycle checkpoints and initiation of apoptosis.
Copyright (c) 2007 International Society for Analytical Cytology.
Figures
Figure 1
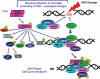
The model of the signaling pathway mediated by ATM in response to DSBs induced by IR, as proposed by Kitagawa et al. (10). Upon induction of DSB the MRE11, RAD50, and NBS1 proteins (MRN complex), as well as BRCA1, are recruited to its site. The MRN complex, when recruited to a DSB, combined with the “structural” change in chromatin resulting from DNA breakage (unwinding and altered torsional stress of the DNA double helix) activate ATM by triggering its auto-phosphorylation, which causes dissociation of the ATM dimer into two enzymatically active monomers, located at some distance from the DSB. After activation, ATM may phosphorylate several nucleoplasmic substrates such as p53, Chk1, Chk2, E2F1. The activated, monomeric ATM is recruited to the DSB when NBS1 and BRCA1 are already at the break site, at which point it phosphorylates several substrates, including NBS1 and SMC1. Phosphorylation of NBS1 targets ATM to Chk1 (11), phosphorylation of SMC1 activates the S-phase checkpoint (10,12), while BRCA1 phosphorylation engages this protein in DSB repair involving either nonhomologous end joining (NHEJ) or homology-directed repair (HR). Phosphorylation of NBS1 is also essential for MRN stimulation of ATM activity towards Chk2 (6). The MRN complex is critical for recruitment of ATM to the site of DSB and in mediating phosphorylation of p53 and H2AX by ATM (6). Phosphorylation of p53 may lead to upregulation of p21 or Bax thereby arresting the cell in G1 or inducing apoptosis, respectively.
Figure 2
Induction of _γ_H2AX and ATM-S1981P expression in HL-60 cells following exposure to UV light. Exponentially growing HL-60 cells untreated (Ctrl) or exposed to 57.5 J/m2 of UVB light were left in culture for 30 or 60 min after the exposure before harvesting, as described (34) The expression of _γ_H2AX or ATM-1981P was measured concurrently with cellular DNA content by flow cytometry, and the data are shown as bivariate _γ_H2AX IF (or ATM-S1981P IF) versus DNA content distributions. The dashed skewed lines represent the maximal level of _γ_H2AX IF or ATM-S1981P IF for over 95% G1- and S-phase cells from the nonirradiated (Ctrl) cultures. The inset in the left panel shows the cellular DNA content frequency histogram of the untreated cells; the cells from the UV irradiated cultures displayed nearly identical histograms. Mitotic cells (M) from untreated cultures show higher expression of _γ_H2AX and ATM-S1981P than G2 cells (21,71) and are marked, respectively, in Ctrl samples.
Figure 3
Effect of topotecan (Tpt) and mitoxantrone (Mxt) on histone H2AX phosphorylation and induction of apoptosis in HL-60 cells. The cells were untreated (0 h) or treated with 150 nM Tpt (top panels) or 0.2 _μ_M Mxt (bottom panels) for 1, 3, or 4 h. _γ_H2AX IF along with DNA content was measured in the untreated cells and 1 h after administration of Tpt or Mxt, by flow cytometry. Apoptotic cells were identified as the cells expressing activated caspase-3 (77) or by the TUNEL assay (78) in the cultures treated with Tpt or Mxt for 3 or 4 h, respectively. The skewed dashed lines represents the upper level of _γ_H2AX expression for 97% of the cells from the untreated (0 h) culture, representing constitutive H2AX phosphorylation (47,63). Based on differences in DNA content, the cells in G1, S, and G2M phases of the cell cycle were identified as shown in the left and right panels. The DNA content frequency histograms of the untreated cells are presented as an inset in the left panels. The plots show an increase (Δ) in mean _γ_H2AX IF, estimated by gating analysis for G1, S, and G2M cell populations, as a function of treatment with different concentration of Tpt or Mxt for 1 h (32,79).
Figure 4
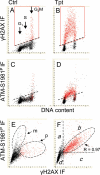
Induction of ATM activation and H2AX phosphorylation in A549 cells treated with Tpt. Untreated (Ctrl, Panels A, C, E) and Tpt-treated (150 nM, 1 h, Panels B, D, F) A549 cells were subjected to immuno-staining to differentially label _γ_H2AX and ATM-S1981P using Alexa Fluor 488 and Alexa Fluor 670 Abs, respectively. Cellular DNA was counterstained with DAPI; the emitted blue, green, and far red fluorescence was measured by three-laser scanning cytometry as described (77,79). As explained in the text, by gating analysis the cells expressing _γ_H2AX were colored red. The regions in Panel F represent the areas on the scatterplot locating the cells that are ATM-S1981P positive and _γ_H2AX negative (a), ATM-S1981P positive and _γ_H2AX positive (b), ATM-S1981P negative and _γ_H2AX positive (c), and ATM-S1981P negative and _γ_H2AX negative (d).
Figure 5
Effect of treatment of TK6 cells with NO-ASA in the absence and presence of NAC, and with NAC alone, on ATM activation and phosphorylation of H2AX. Bivariate distributions representing cellular DNA content versus _γ_H2AX IF or versus ATM-S1981P IF of TK6 cells untreated (Ctrl), treated with 5 _μ_M of NO-ASA alone, or with 5 _μ_M of NO-ASA in the presence of 50 mM NAC. NAC was included 20 min prior to administration of NO-ASA; exposure to NO-ASA or NO-ASA plus NAC was for 1 h. The skewed dashed line represents the upper level of _γ_H2AX expression for 97% G1 and S-phase cells from the untreated (Ctrl) culture, representing constitutive H2AX phosphorylation (47,48,63). Mitotic cells (M) express ATM-1981P and _γ_H2AX constitutively and they are marked by the rectangular dashed line boundaries. The DNA content frequency histogram of the cells is shown in the inset in the left (Ctrl) panel. Note an induction of ATM-S1981P and _γ_H2AX primarily in S-phase cells after treatment with NO-ASA, and attenuation of this effect in the presence of NAC.
Figure 6
Effect of treatment of HL-60 cells with aphidicolin (Aph) or hydroxyurea (Hxu) on ATM activation and H2AX phosphorylation. Bivariate (ATM-S1981P IF or _γ_H2AX IF vs. DNA content) distributions representing untreated cells (Ctrl) and cells treated with 0.5 mM Hxu for 2 h, or with 4 _μ_M Aph for 2 h (23). Subpopulations of cells in G1, S, and G2M can be distinguished based on differences in DNA content, as shown in the respective left panels. Apoptotic cells (Ap), identified by fluorescence microscopy (23), are characterized by markedly increased ATM-S1981P as well as _γ_H2AX IF; they are located within the oval dashed gates. The dashed-line thresholds show the upper limit of ATM-S1981P or H2AX IF for 97% of the cells from the untreated (Ctrl) cultures, respectively. A DNA content histogram of the untreated cells is shown in the Ctrl _γ_H2AX versus DNA content panel as an inset.
Figure 7
Induction of ATM activation and H2AX phosphorylation by exposure of cells to cigarette smoke. A549 cells were “mock” treated (Ctrl) or exposed to cigarette smoke for 5 or 20 min, as described (35,90). The cells were then incubated in culture for 1 h, fixed and immunostained for ATM-S1981P or _γ_H2AX; DNA was counterstained with DAPI, cellular fluorescence was measured by laser scanning cytometry (LSC). The dashed-line thresholds show the upper limit of ATM-S1981P or _γ_H2AX IF for 97% of the cells from the mock-treated (Ctrl) cultures, respectively. The inset in the left panel shows the DNA content histogram of the untreated cells prior to exposure; cell treatment with smoke followed by 1 h incubation did not significantly affect the cell cycle distribution (not shown).
Figure 8
Kinetics of cell cycle progression revealed by UV-induced photolysis of DNA containing incorporated BrdU and reported by H2AX phosphorylation in relation to cell cycle phase. Expression of _γ_H2AX in HeLa cells pulse-labeled with BrdU and then incubated for 2, 8, or 12 h prior to UV irradiation; left panel shows BrdU labeled but not irradiated cells. Following UV irradiation, the cells were cultured for an additional 1 h, then fixed, their _γ_H2AX was detected immunocytochemically, the DNA was counterstained with DAPI, and cellular fluorescence was measured by laser scanning cytometer, as described (102). Insets show DNA content frequency histograms from the respective cultures. Note that after 8 h the BrdU-labeled cells are predominantly in G2M and the very first labeled cells already appear in G1. The frequency of BrdU-labeled cells in G1 is distinctly higher 12 h after pulse-labeling with BrdU compared with 8 h, while fewer labeled cells remain in G2M.
Figure 9
Different levels of H2AX phosphorylation and ATM activation in non-apoptotic and apoptotic HL-60 cells treated with the DNA polymerase-α inhibitor aphidicolin. HL-60 cells were untreated (Ctrl) or treated with 4 _μ_M aphidicolin (Aph) for 2 h in culture. The bivariate distributions show expression of _γ_H2AX or ATM-S1981P versus DNA content, respectively. The replication stress caused by Aph results in H2AX phosphorylation in the absence of ATM activation (23). The Aph-induced _γ_H2AX expression is primarily limited to the S-phase cells, with the early-S cells most affected. The cells marked enS most likely were entering S phase (initiating DNA replication) during the 2-h interval after administration of Aph. The dashed-line thresholds show the upper limit of _γ_H2AX or ATM-S1981P IF for 95% of the cells from the untreated (Ctrl) cultures, respectively. Treatment with Aph induces apoptosis. Apoptotic cells (Ap) are characterized by markedly higher _γ_H2AX and ATM-S1981P IF compared with nonapoptotic cells. The inset shows a DNA content frequency histogram from the untreated culture; the 2-h treatment with Aph had minimal effect on the DNA content distribution (not shown; see Ref. 23).
Figure 10
Correlation between induction of _γ_H2AX, caspase-3 activation and cell cycle position in HL-60 cells treated with Tpt for 1 or 3h. The respective scatterplots show the bivariate distributions of _γ_H2AX IF versus cellular DNA content, or versus expression of activated caspase-3 (caspase-3*). Each of these parameters was measured using fluorochromes of different color (_γ_H2AX, long-red; DNA, blue; caspase-3*, green) excited by different lasers; fluorescence was measured by LSC, as described (32,109). IF of _γ_H2AX and of caspase-3* is presented as maximal pixel (MP). The thresholds (dashed skewed lines) represent the upper level of _γ_H2AX IF for 95% of the untreated (Ctrl) cells. Note that apoptotic cells (Ap) seen after treatment with Tpt for 3 h are characterized by very high expression of _γ_H2AX and also by expression of activated caspase. The cells with primary DSBs induced by Tpt (prDSBs) show elevated expression of _γ_H2AX but no caspase-3 activation.
Similar articles
- Assessment of ATM phosphorylation on Ser-1981 induced by DNA topoisomerase I and II inhibitors in relation to Ser-139-histone H2AX phosphorylation, cell cycle phase, and apoptosis.
Kurose A, Tanaka T, Huang X, Halicka HD, Traganos F, Dai W, Darzynkiewicz Z. Kurose A, et al. Cytometry A. 2005 Nov;68(1):1-9. doi: 10.1002/cyto.a.20186. Cytometry A. 2005. PMID: 16184611 - ATM activation and histone H2AX phosphorylation as indicators of DNA damage by DNA topoisomerase I inhibitor topotecan and during apoptosis.
Tanaka T, Kurose A, Huang X, Dai W, Darzynkiewicz Z. Tanaka T, et al. Cell Prolif. 2006 Feb;39(1):49-60. doi: 10.1111/j.1365-2184.2006.00364.x. Cell Prolif. 2006. PMID: 16426422 Free PMC article. - Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis.
Huang X, Halicka HD, Traganos F, Tanaka T, Kurose A, Darzynkiewicz Z. Huang X, et al. Cell Prolif. 2005 Aug;38(4):223-43. doi: 10.1111/j.1365-2184.2005.00344.x. Cell Prolif. 2005. PMID: 16098182 Free PMC article. Review. - Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants.
Tanaka T, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Tanaka T, et al. Cell Cycle. 2006 Sep;5(17):1940-5. doi: 10.4161/cc.5.17.3191. Epub 2006 Sep 1. Cell Cycle. 2006. PMID: 16940754 Free PMC article. Review.
Cited by
- Targeting DNA repair with aphidicolin sensitizes primary chronic lymphocytic leukemia cells to purine analogs.
Starczewska E, Beyaert M, Michaux L, Vekemans MC, Saussoy P, Bol V, Arana Echarri A, Smal C, Van Den Neste E, Bontemps F. Starczewska E, et al. Oncotarget. 2016 Jun 21;7(25):38367-38379. doi: 10.18632/oncotarget.9525. Oncotarget. 2016. PMID: 27223263 Free PMC article. - Cidofovir is active against human papillomavirus positive and negative head and neck and cervical tumor cells by causing DNA damage as one of its working mechanisms.
Mertens B, Nogueira T, Stranska R, Naesens L, Andrei G, Snoeck R. Mertens B, et al. Oncotarget. 2016 Jul 26;7(30):47302-47318. doi: 10.18632/oncotarget.10100. Oncotarget. 2016. PMID: 27331622 Free PMC article. - Anti-cancer effects of newly developed chemotherapeutic agent, glycoconjugated palladium (II) complex, against cisplatin-resistant gastric cancer cells.
Tanaka M, Kataoka H, Yano S, Ohi H, Kawamoto K, Shibahara T, Mizoshita T, Mori Y, Tanida S, Kamiya T, Joh T. Tanaka M, et al. BMC Cancer. 2013 May 14;13:237. doi: 10.1186/1471-2407-13-237. BMC Cancer. 2013. PMID: 23672493 Free PMC article. - Kinetics of the UV-induced DNA damage response in relation to cell cycle phase. Correlation with DNA replication.
Zhao H, Traganos F, Darzynkiewicz Z. Zhao H, et al. Cytometry A. 2010 Mar;77(3):285-93. doi: 10.1002/cyto.a.20839. Cytometry A. 2010. PMID: 20014310 Free PMC article. - DNA damage signaling is activated during cancer progression in human colorectal carcinoma.
Oka K, Tanaka T, Enoki T, Yoshimura K, Ohshima M, Kubo M, Murakami T, Gondou T, Minami Y, Takemoto Y, Harada E, Tsushimi T, Li TS, Traganos F, Darzynkiewicz Z, Hamano K. Oka K, et al. Cancer Biol Ther. 2010 Feb;9(3):246-52. doi: 10.4161/cbt.9.3.10751. Epub 2010 Feb 25. Cancer Biol Ther. 2010. PMID: 20023412 Free PMC article.
References
- Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. - PubMed
- Helt CE, Cliby WA, Keng PC, Bambara RA, O'Reilly MA. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J Biol Chem. 2005;280:1186–1192. - PubMed
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular auto-phosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
- Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. - PubMed
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous