Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes - PubMed (original) (raw)
Comparative Study
. 2007 Sep;81(18):9922-31.
doi: 10.1128/JVI.00988-07. Epub 2007 Jul 11.
Affiliations
- PMID: 17626097
- PMCID: PMC2045393
- DOI: 10.1128/JVI.00988-07
Comparative Study
Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes
Jessica L Smith et al. J Virol. 2007 Sep.
Abstract
Papillomaviruses are species-specific and epitheliotropic DNA viruses that cause tumors in their natural hosts. Certain infections with genital human papillomavirus (HPV) types are causally related to cervical cancer development. Most papillomaviruses are thought to infect cells via a clathrin-dependent pathway, yet no studies have determined the entry route in permissive host epithelial cells. Employing fluorescently labeled and native virions, we tested the effects of dominant-negative and biochemical inhibitors of cellular endocytosis pathways. Infections of human keratinocytes, a natural host cell type for HPVs, were assessed visually and by infectious entry assays. We found that HPV type 31 (HPV31) entry and initiation of early infection events require both caveolin 1 and dynamin 2 and occur independently of clathrin-mediated endocytosis. Treatment with chlorpromazine and filipin had opposing effects on HPV31 and HPV16 infection. HPV31 entry was remarkably slow, with a half-time of approximately 14 h, whereas the entry half-time of HPV16 was 4 h. Consistent with a caveola-mediated entry pathway for HPV31, the virions associated with detergent-resistant lipid rafts. During a 16-h microscopic tracking of HPV31 and HPV16 virions, no colocalization of the two viral types was observed. These data suggest that HPV31 and HPV16 virions use distinct routes for host epithelial cell entry.
Figures
FIG. 1.
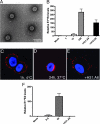
HPV31 virions produced by a transfection-based method display normal morphology and infectivity properties. (A) Transfection-derived HPV31 virions visualized by TEM. (B) HaCaT cells were exposed to increasing doses of HPV31 (1, 10, and 100 vge/cell) as described in Materials and Methods. Monoclonal antibodies H31.A6 and H16.D9 were diluted in media and added to cells immediately following viral attachment. Total RNAs were subjected to RT-qPCR in triplicate to quantify spliced HPV31-E1̂E4 transcripts indicative of infection (48 h p.i.). Error bars represent SEM. (C) AF594-labeled HPV31 (red) was bound to HKs at 10,000 vge/cell and visualized after 1 h at 4°C, after incubation at 37°C for 24 h (D), or after incubation at 37°C for 24 h in the presence of H31.A6 neutralizing antibody (E). Coverslips were mounted in the presence of DAPI to visualize nuclei. (F) HaCaT cells were inoculated with AF594-labeled HPV31 at the indicated doses; neutralizing antibody H31.A6 was used as in panels B and E. Infection was quantified as in panel B.
FIG. 2.
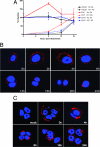
Time course of HPV31 internalization. (A) Organotypic culture-derived or transfection-derived HPV31 virions were bound to HaCaT (black boxes), Ect1 (red triangles), or End1 (blue circles) cells for 1 h at 4°C at a dose of 50 vge/cell, and cells were washed and refed with regular growth media. At the indicated times postattachment, cells were treated with H31.A6 (dashed lines) or refed with new media (solid lines). The cells were harvested at 48 h p.i., and RNA was analyzed in triplicate by RT-qPCR for HPV31 E1̂E4 levels; values were normalized to the average of the untreated controls at 0 h. Error bars represent SEM. Ab, antibody. (B) AF594-labeled HPV31 was bound to HaCaT cells on glass coverslips at 10,000 vge/cell for 1 h at 4°C. Cells were washed, refed with regular growth medium, and shifted to 37°C. At indicated times postattachment, cells were fixed and stained with DAPI to visualize nuclei. (C) AF594-labeled HPV16 was bound to HaCaT cells on glass coverslips at 10,000 vge/cell for 1 h at 4°C. Cells were washed, refed with regular growth medium, and shifted to 37°C. At indicated times postattachment, cells were fixed and stained with DAPI to visualize nuclei.
FIG. 3.
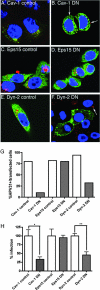
Effects of dominant-negative endocytosis inhibitors on HPV31 entry and early transcription. (A to F) HaCaT cells transfected with GFP-tagged dominant-negative inhibitors of endocytosis and their corresponding controls. At 48 h posttransfection, AF594-HPV31 virions were bound to cells for 1 h at 4°C and cells were washed, refed with regular growth medium, and placed at 37°C. Cells were fixed and stained with DAPI 24 h after the 37°C shift to promote virus internalization. (A and B) Wild-type caveolin 1-GFP (Cav-1 control) and dominant-negative caveolin 1 (Cav-1 DN). (C and D) Wild-type Esp15 (Eps15 control) and Eps15 dominant-negative Eps15 (Eps15 DN). (E and F) Wild-type dynamin 2 (Dyn-2 control) and dominant negative dynamin 2 (Dyn-2 DN). Results are representative of three replicate transfection/infection experiments. (G) Quantification of HPV31 particle entry into transfected cells was performed by blinded evaluation of internalization state of AF594-HPV31 in a total of 100 transfected cells (expressing GFP). (H) Triplicate cultures of transfected HaCaT cells were infected 48 h posttransfection with HPV31 virions at 50 vge/cell. RNA isolated 48 h p.i. was quantified in triplicate for HPV31 E1̂E4 transcript levels by RT-qPCR. Error bars represent SEM, and statistical significance was achieved with P < 0.05 (*) and P < 0.01 (**).
FIG. 4.
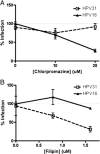
Effects of biochemical endocytic inhibitors on HPV31 and HPV16 infection of HKs. HaCaT cells were pretreated with increasing concentrations of chlorpromazine (A [0, 10, and 20 μM]) or filipin (B [0, 0.8, and 1.6 μM]) for 1 h at 37°C. HPV31 (open boxes, dashed lines) or HPV16 (triangles, solid lines) virions at 50 vge/cell were bound for 1 h at 4°C to promote viral attachment. Cells were then washed, refed with medium/inhibitor, and placed at 37°C. RNA isolated at 48 h p.i. was quantified for HPV31 E1̂E4 transcript levels by RT-qPCR. Infections were performed in duplicate, and RT-qPCR was performed in triplicate, with error bars representing SEM.
FIG. 5.
HPV31 associates with detergent-resistant lipid rafts. (A) AF594-labeled ligands (CT-B, TF, HPV31, or HPV16) were bound to HaCaT cells for 1 h at 4°C and then washed and refed with regular growth medium before incubation at 37°C for 30 min. MβCD treatment for 30 min at 37°C was performed to disrupt cholesterol (right column). Cells were subjected to a control PBS wash (left column) or TX-100 wash (middle and right columns) on ice to remove detergent-soluble membranes. Cells were then fixed and stained with DAPI to visualize nuclei. (B) AF594-HPV31 was bound to cells for 1 h at 4°C and then washed, refed with regular growth medium, and placed at 37°C. At indicated times postattachment, cells were subjected to a control PBS wash (left column) or TX-100 wash (right column) before fixation. (C) HPV31 was bound to HaCaT cells for 1 h at 4°C, and cells were washed and refed with normal growth medium before incubation at 37°C for 30 min in the presence or absence of MβCD. Cells were then lysed, and the samples were subjected to centrifugation on a discontinuous density gradient. Fractions were collected from the top and from pelleted protein (P) and analyzed for HPV31 L1 protein by Western blotting.
FIG. 6.
HPV31 and HPV16 fail to colocalize during HK entry. AF594-HPV16 (red) and AF488-HPV31 (green) virions (each at 10,000 vge/cell) were added to HaCaT cells for 1 h at 4°C, and cells were washed, refed with regular growth medium, and placed at 37°C. At the indicated time points, cells were fixed, DAPI stained, and visualized by confocal microscopy. Lower panels are higher magnifications of boxed areas in each upper panel.
Similar articles
- Clathrin- and caveolin-independent entry of human papillomavirus type 16--involvement of tetraspanin-enriched microdomains (TEMs).
Spoden G, Freitag K, Husmann M, Boller K, Sapp M, Lambert C, Florin L. Spoden G, et al. PLoS One. 2008 Oct 2;3(10):e3313. doi: 10.1371/journal.pone.0003313. PLoS One. 2008. PMID: 18836553 Free PMC article. - Caveolin-1-dependent infectious entry of human papillomavirus type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating.
Smith JL, Campos SK, Wandinger-Ness A, Ozbun MA. Smith JL, et al. J Virol. 2008 Oct;82(19):9505-12. doi: 10.1128/JVI.01014-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667513 Free PMC article. - Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin a sensitive.
Laniosz V, Dabydeen SA, Havens MA, Meneses PI. Laniosz V, et al. J Virol. 2009 Aug;83(16):8221-32. doi: 10.1128/JVI.00576-09. Epub 2009 Jun 3. J Virol. 2009. PMID: 19494002 Free PMC article. - The evolving field of human papillomavirus receptor research: a review of binding and entry.
Raff AB, Woodham AW, Raff LM, Skeate JG, Yan L, Da Silva DM, Schelhaas M, Kast WM. Raff AB, et al. J Virol. 2013 Jun;87(11):6062-72. doi: 10.1128/JVI.00330-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536685 Free PMC article. Review. - Biology and pathological associations of the human papillomaviruses: a review.
Cheah PL, Looi LM. Cheah PL, et al. Malays J Pathol. 1998 Jun;20(1):1-10. Malays J Pathol. 1998. PMID: 10879257 Review.
Cited by
- Papillomavirus Infectious Pathways: A Comparison of Systems.
Biryukov J, Meyers C. Biryukov J, et al. Viruses. 2015 Aug 4;7(8):4303-25. doi: 10.3390/v7082823. Viruses. 2015. PMID: 26247955 Free PMC article. Review. - Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus.
Selinka HC, Florin L, Patel HD, Freitag K, Schmidtke M, Makarov VA, Sapp M. Selinka HC, et al. J Virol. 2007 Oct;81(20):10970-80. doi: 10.1128/JVI.00998-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686860 Free PMC article. - The role of NH4Cl and cysteine proteases in Human Papillomavirus type 16 infection.
Dabydeen SA, Meneses PI. Dabydeen SA, et al. Virol J. 2009 Jul 20;6:109. doi: 10.1186/1743-422X-6-109. Virol J. 2009. PMID: 19619315 Free PMC article. - Concepts of papillomavirus entry into host cells.
Day PM, Schelhaas M. Day PM, et al. Curr Opin Virol. 2014 Feb;4:24-31. doi: 10.1016/j.coviro.2013.11.002. Epub 2013 Dec 14. Curr Opin Virol. 2014. PMID: 24525291 Free PMC article. Review. - Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes.
Dziduszko A, Ozbun MA. Dziduszko A, et al. J Virol. 2013 Jul;87(13):7502-15. doi: 10.1128/JVI.00519-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637395 Free PMC article.
References
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. - PubMed
- Bosch, F., M. Manos, N. Munoz, M. Sherman, A. Jansen, J. Peto, M. Schiffman, V. Moreno, R. Kurman, K. Shah, et al. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87:796-802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI 07538/AI/NIAID NIH HHS/United States
- CA 085747/CA/NCI NIH HHS/United States
- T32 AI007538/AI/NIAID NIH HHS/United States
- R01 CA085747/CA/NCI NIH HHS/United States
- F32 CA 123842/CA/NCI NIH HHS/United States
- F32 CA123842/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical