Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors - PubMed (original) (raw)
. 2007 Jul 11;27(28):7459-68.
doi: 10.1523/JNEUROSCI.1483-07.2007.
Victoria F Turek, Maria C Almeida, Jeffrey J Burmeister, Daniela L Oliveira, Jennifer L Roberts, Anthony W Bannon, Mark H Norman, Jean-Claude Louis, James J S Treanor, Narender R Gavva, Andrej A Romanovsky
Affiliations
- PMID: 17626206
- PMCID: PMC6672610
- DOI: 10.1523/JNEUROSCI.1483-07.2007
Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors
Alexandre A Steiner et al. J Neurosci. 2007.
Abstract
An involvement of the transient receptor potential vanilloid (TRPV) 1 channel in the regulation of body temperature (T(b)) has not been established decisively. To provide decisive evidence for such an involvement and determine its mechanisms were the aims of the present study. We synthesized a new TRPV1 antagonist, AMG0347 [(E)-N-(7-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl)-3-(2-(piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)acrylamide], and characterized it in vitro. We then found that this drug is the most potent TRPV1 antagonist known to increase T(b) of rats and mice and showed (by using knock-out mice) that the entire hyperthermic effect of AMG0347 is TRPV1 dependent. AMG0347-induced hyperthermia was brought about by one or both of the two major autonomic cold-defense effector mechanisms (tail-skin vasoconstriction and/or thermogenesis), but it did not involve warmth-seeking behavior. The magnitude of the hyperthermic response depended on neither T(b) nor tail-skin temperature at the time of AMG0347 administration, thus indicating that AMG0347-induced hyperthermia results from blockade of tonic TRPV1 activation by nonthermal factors. AMG0347 was no more effective in causing hyperthermia when administered into the brain (intracerebroventricularly) or spinal cord (intrathecally) than when given systemically (intravenously), which indicates a peripheral site of action. We then established that localized intra-abdominal desensitization of TRPV1 channels with intraperitoneal resiniferatoxin blocks the T(b) response to systemic AMG0347; the extent of desensitization was determined by using a comprehensive battery of functional tests. We conclude that tonic activation of TRPV1 channels in the abdominal viscera by yet unidentified nonthermal factors inhibits skin vasoconstriction and thermogenesis, thus having a suppressive effect on T(b).
Figures
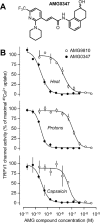
Figure 1.
Chemical structure and in vitro pharmacology of AMG0347. A, Structural formula of AMG0347 as confirmed by spectral analysis (supplemental Materials and Methods, available at
as supplemental material). B, Concentration-dependent effects of AMG0347 on the activation of the TRPV1 channel by heat, protons, and capsaicin. For comparison, the effects of AMG9810 on channel activation by the same stimuli are shown. Channel activation was assessed based on the uptake of 45Ca2+ by cultured Chinese hamster ovary cells stably transfected with the rat TRPV1 channel. The 45Ca2+ uptake assay was conducted three times for each AMG compound–stimulus combination.
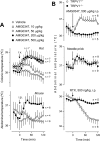
Figure 2.
AMG0347 produces hyperthermia in wild-type rats and mice but not in TRPV1-deficient mice. A, Effects of intravenous AMG0347 (doses indicated) or its vehicle on the colonic (deep body) temperature of rats at a neutral Ta of 28°C and on the abdominal (deep body) temperature of mice at a neutral Ta of 31°C. B, Effects of AMG0347, abdominal needle prick, or RTX on the abdominal temperature of TRPV1+/+ (wild-type) and _TRPV1_−/− (TRPV1-deficient) mice. When the stimulus was expected to result in hyperthermia (AMG0347 administration and needle prick), the experiment was conducted at a neutral Ta (31°C); when the stimulus was expected to result in hypothermia (RTX), the experiment was performed at a subneutral Ta (26°C). The number of animals in each group (n) is indicated.
Figure 3.
AMG0347 hyperthermia is brought about by activation of autonomic thermoeffectors and is independent of basal Tb and Tsk. A, Effects of AMG0347 or its vehicle on the colonic temperature, heat loss index, and oxygen consumption of rats at Ta of 28°C (upper end of the TNZ), 24°C (lower end of the TNZ), or 17°C (below the lower end of the TNZ). Colonic temperature is an index of deep Tb, the heat loss index is an index of skin vasodilation, and oxygen consumption is an index of thermogenesis. B, Results of a linear correlation analysis between the magnitude of AMG0347-induced hyperthermia and the values of either Tb or Tsk immediately before AMG0347 administration. Based on the data presented in A.
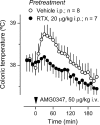
Figure 4.
AMG0347 hyperthermia is absent in rats with localized intra-abdominal TRPV1 desensitization. The colonic temperature response of rats pretreated with RTX (20 μg/kg, i.p.) or its vehicle to administration of AMG0347 at thermoneutrality (Ta of 24°C) is shown.
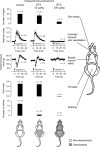
Figure 5.
Sites of TRPV1 desensitization in RTX-pretreated rats. The sensitivity of TRPV1 channels in different bodily compartments was determined in vehicle-pretreated rats, in rats pretreated with a low desensitizing dose (20 μg/kg, i.p.) of RTX, and in rats pretreated with a high dose (200 μg/kg, i.p.) of RTX. Five tests were performed: the eye-wiping test (determines the sensitivity of TRPV1 channels in the eye); the centrally induced tail-vasodilation test (determines the sensitivity of TRPV1 channels in the brain); the Bezold–Jarisch reflex test (determines the sensitivity of TRPV1 channels in the heart and lungs); the hot-plate test (assesses the sensitivity of TRPV1 channels in the skin, primarily of the paws); and the writhing test (determines the sensitivity of TRPV1 channels in the peritoneal cavity). For methodological details, see Materials and Methods. The results of all tests are summarized in the schematics at the bottom.
Comment in
- Thermoregulation: channels that are cool to the core.
Montell C, Caterina MJ. Montell C, et al. Curr Biol. 2007 Oct 23;17(20):R885-7. doi: 10.1016/j.cub.2007.08.016. Curr Biol. 2007. PMID: 17956748 Review.
Similar articles
- Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis.
Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Garami A, et al. J Neurosci. 2011 Feb 2;31(5):1721-33. doi: 10.1523/JNEUROSCI.4671-10.2011. J Neurosci. 2011. PMID: 21289181 Free PMC article. - Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats.
Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, Lee D, Louis JC, Magal E, Manning BH, Rubino J, Surapaneni S, Tamayo N, Wang T, Wang J, Wang J, Wang W, Youngblood B, Zhang M, Zhu D, Norman MH, Gavva NR. Lehto SG, et al. J Pharmacol Exp Ther. 2008 Jul;326(1):218-29. doi: 10.1124/jpet.107.132233. Epub 2008 Apr 17. J Pharmacol Exp Ther. 2008. PMID: 18420600 - The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation.
Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. Gavva NR, et al. J Neurosci. 2007 Mar 28;27(13):3366-74. doi: 10.1523/JNEUROSCI.4833-06.2007. J Neurosci. 2007. PMID: 17392452 Free PMC article. - The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not.
Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. Romanovsky AA, et al. Pharmacol Rev. 2009 Sep;61(3):228-61. doi: 10.1124/pr.109.001263. Epub 2009 Sep 11. Pharmacol Rev. 2009. PMID: 19749171 Free PMC article. Review. - Thermoregulation: channels that are cool to the core.
Montell C, Caterina MJ. Montell C, et al. Curr Biol. 2007 Oct 23;17(20):R885-7. doi: 10.1016/j.cub.2007.08.016. Curr Biol. 2007. PMID: 17956748 Review.
Cited by
- Central control of body temperature.
Morrison SF. Morrison SF. F1000Res. 2016 May 12;5:F1000 Faculty Rev-880. doi: 10.12688/f1000research.7958.1. eCollection 2016. F1000Res. 2016. PMID: 27239289 Free PMC article. Review. - Inhibition of capsaicin-driven nasal hyper-reactivity by SB-705498, a TRPV1 antagonist.
Holland C, van Drunen C, Denyer J, Smart K, Segboer C, Terreehorst I, Newlands A, Beerahee M, Fokkens W, Tsitoura DC. Holland C, et al. Br J Clin Pharmacol. 2014 May;77(5):777-88. doi: 10.1111/bcp.12219. Br J Clin Pharmacol. 2014. PMID: 23909699 Free PMC article. Clinical Trial. - Transient receptor potential vanilloid 1: A potential therapeutic target for the treatment of osteoarthritis and rheumatoid arthritis.
Liao Z, Umar M, Huang X, Qin L, Xiao G, Chen Y, Tong L, Chen D. Liao Z, et al. Cell Prolif. 2024 Mar;57(3):e13569. doi: 10.1111/cpr.13569. Epub 2023 Nov 23. Cell Prolif. 2024. PMID: 37994506 Free PMC article. Review. - Central neural regulation of brown adipose tissue thermogenesis and energy expenditure.
Morrison SF, Madden CJ, Tupone D. Morrison SF, et al. Cell Metab. 2014 May 6;19(5):741-756. doi: 10.1016/j.cmet.2014.02.007. Epub 2014 Mar 13. Cell Metab. 2014. PMID: 24630813 Free PMC article. Review. - TRPV1 activation is not an all-or-none event: TRPV1 partial agonism/antagonism and its regulatory modulation.
Blumberg PM, Pearce LV, Lee J. Blumberg PM, et al. Curr Top Med Chem. 2011;11(17):2151-8. doi: 10.2174/156802611796904825. Curr Top Med Chem. 2011. PMID: 21671879 Free PMC article. Review.
References
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci. 2006b;23:3359–3367. - PubMed
- Aviado DM, Guevara-Aviado D. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann NY Acad Sci. 2001;940:48–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 AA015660/AA/NIAAA NIH HHS/United States
- R01 NS041233/NS/NINDS NIH HHS/United States
- F32 AA-015660/AA/NIAAA NIH HHS/United States
- R01 NS-41233/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources