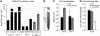Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution - PubMed (original) (raw)
Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution
Kamil Růzicka et al. Plant Cell. 2007 Jul.
Abstract
In plants, each developmental process integrates a network of signaling events that are regulated by different phytohormones, and interactions among hormonal pathways are essential to modulate their effect. Continuous growth of roots results from the postembryonic activity of cells within the root meristem that is controlled by the coordinated action of several phytohormones, including auxin and ethylene. Although their interaction has been studied intensively, the molecular and cellular mechanisms underlying this interplay are unknown. We show that the effect of ethylene on root growth is largely mediated by the regulation of the auxin biosynthesis and transport-dependent local auxin distribution. Ethylene stimulates auxin biosynthesis and basipetal auxin transport toward the elongation zone, where it activates a local auxin response leading to inhibition of cell elongation. Consistently, in mutants affected in auxin perception or basipetal auxin transport, ethylene cannot activate the auxin response nor regulate the root growth. In addition, ethylene modulates the transcription of several components of the auxin transport machinery. Thus, ethylene achieves a local activation of the auxin signaling pathway and regulates root growth by both stimulating the auxin biosynthesis and by modulating the auxin transport machinery.
Figures
Figure 1.
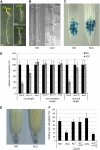
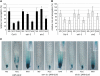
Ethylene Inhibits Root Growth through Regulation of Cell Elongation. (A) Seedlings germinated on ethylene precursor ACC or synthetic auxin NAA exhibit comparably reduced root growth. NAA, 1-naphthylacetic acid. (B) ACC strongly inhibits elongation of wild-type epidermal cells, as quantified in (D). (C) ACC does not affect cell cycle activity of the root meristem, as monitored by the proCYCB;1-GUSDB reporter. (D) ACC inhibits total root length and epidermal cell length of control seedlings (ACC treated versus untreated wild-type; P < 0.05, analysis of variance [ANOVA]). Root and cell lengths of _etr1-3_ and _ein2-1_ are ethylene resistant (P > 0.05, ANOVA). Root meristem length is not affected by ACC in the wild type nor in the mutants (P > 0.05, ANOVA). 100% corresponds to 14.2 ± 1.76 mm of the wild type, 13.7 ± 2.03 mm of etr1-3, and 16.8 ± 1.52 mm of ein2 root length. (E) Columella differentiation in wild-type roots is not affected by ACC treatment. Starch granules are visualized by Lugol staining. (F) ACC inhibits root elongation. The inhibitor of the ethylene perception (Ag+) (P > 0.05, ANOVA) but not that of the ethylene biosynthesis (AVG) (P < 0.05, ANOVA) interferes with simultaneously applied ACC in root elongation. Reduction of the endogenous ethylene level by AVG treatment results in significantly longer roots than those of the control (P < 0.05, ANOVA). Five-day-old seedlings were grown in the presence of 200 nM NAA, 1 μM ACC, 0.2 μM AVG, and 20 μM AgNO3. Data are means ±
sd
.
Figure 2.
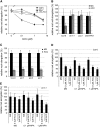
Ethylene Insensitivity in Mutants with Abolished Basipetal Auxin Transport. (A) Lines affected in the root basipetal auxin transport (eir1-1, aux1-T, and 35S-PIN1) exhibit ethylene-resistant root growth. The ethylene effects on the wild type compared with eir1-1, aux1-T, or 35S-PIN1 were significantly different (P < 0.05, ANOVA). 100% corresponds to 12.5 ± 1.95 mm of the wild type, 9.9 ± 2.33 mm of eir1-1, 9.1 ± 1.99 mm of aux1, and 13.0 ± 2.39 mm of 35S-PIN1 root length. For clarity, depicted data are mean ±
se
. (B) ACC does not inhibit the elongation of the epidermal cells in eir1-1, aux1-T, and 35S-PIN1 (P < 0.05, ANOVA). **(C)** Auxin efflux carrier mutants _pin1-3_, _pin4-1_, and _pin7-2_ (with normal root basipetal auxin transport) are ethylene sensitive. The ACC effect on _pin1_, _pin4_, and _pin7_ roots was not significantly different from that on the wild type (P > 0.05, ANOVA). 100% corresponds to 24.4 ± 2.48 mm of the wild type, 23.3 ± 2.90 mm of pin1, 25.3 ± 2.35 mm of pin4, and 26.1 ± 2.71 mm pin7 root length. (D) Inhibitor of polar auxin transport (NPA) at any concentration does not interfere with ethylene sensitivity of the root growth. No significant difference was found between the root lengths of seedlings treated with ACC and simultaneously with NPA and ACC (P > 0.05, ANOVA). (E) ACC sensitivity of eir1-1 roots is increased by NPA treatment. The root length of eir1-1 treated simultaneously with 1 μM NPA and 1 μM ACC was significantly shorter than that of seedlings germinated on 1 μM ACC (P < 0.05, ANOVA). Seedlings were grown, if not specified differently, in the presence of 1 μM ACC. Data are mean ±
sd
(except in [A]).
Figure 3.
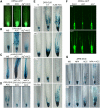
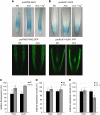
Induction of DR5 Reporter Expression by Ethylene in the Root Meristem and the Root Elongation Zone. (A) to (C) Auxin-sensitive reporters DR5rev-GFP (A), DR5-GUS (B), and proIAA2-GUS (C) are ectopically expressed after ACC treatment. Ag+ (ethylene perception inhibitor) blocks ACC-triggered ectopic expression. Prolonged GUS staining (B) reveals an increased auxin response in the outer layers of the root meristem (lateral root cap and epidermis) and in the elongating cells. The arrows point to the tissues with the ectopic DR5 signal. (D) ACC treatment induces ectopic expression of the DR5-GUS reporter in the wild type but not in the etr1-3 and ein2-1 mutant backgrounds. (E) ACC treatment induces ectopic expression of the DR5-GUS reporter in the wild type but not in the eir1-1, aux1-T, and 35S-PIN1 backgrounds. Higher doses of ACC (5 μM) stimulate DR5 expression in the epidermal cells of the meristem and partially also in the elongation zone of mutant roots. (F) Confocal sections show that the ACC-induced ectopic expression of the DR5rev-GFP reporter is restricted to the lateral root cap and the epidermis of the root meristem in the wild type and a comparable expression pattern of the reporter in the pin1-3 and pin7-2 background. (G) Inhibitor of the polar auxin transport (NPA) does not interfere with ACC-induced ectopic expression of DR5-GUS in the wild-type background. Simultaneous application of ACC and NPA induces the ectopic expression of DR5-GUS in the eir1-1 mutant. Five-day-old seedlings were grown, if not specified differently, in the presence of 1 μM ACC, 20 μM AgNO3, and 1 μM NPA.
Figure 4.
Auxin- and Ethylene-Resistant Root Growth of Auxin Signaling Mutants. (A) Ectopic expression of HS-axr3 induced by heat shock leads to auxin- and ethylene-insensitive root growth. Statistically significant difference was confirmed between nontreated and heat shock–treated seedlings germinated either on NAA or ACC (P < 0.05, ANOVA). 100% corresponds to 8.8 ± 0.99 mm and 10.4 ± 1.06 mm of non-heat-shocked or to 9.2 ± 0.92 mm and 3.6 ± 0.81 mm of heat-shocked wild type and HS-axr3 root growth following heat shock, respectively. (B) NAA- and ACC-dependent inductions of DR5-GUS in the epidermal cells of the root meristem and cells of the elongation zone do not occur in the HS-axr3 line after the heat shock. (C) The tir1-1 mutant exhibits auxin- and ethylene-insensitive root growth (P < 0.05, ANOVA; validated for 50, 100, and 200 nM NAA and 0.3 and 1 μM ACC). 100% corresponds to 23.1 ± 2.37 mm of the wild type and 22.7 ± 2.28 mm of tir1-1 root length. For clarity, depicted data are means ±
se
. (D) Terfestatin A (Trf), a chemical inhibitor of auxin response, interferes with auxin- and ethylene-mediated ectopic upregulation of the DR5-GUS reporter in roots. Seedlings were incubated for 24 h in liquid medium containing, as specified, 50 μM Trf, 5 μM IAA, or 1 μM ACC. (E) shy2-2 (carrying the _IAA3-_stabilized allele) exhibits strong auxin (P < 0.05, ANOVA) but not significant ethylene resistance (P > 0.05, ANOVA) compared with the control. 100% corresponds to 10.6 ± 1.29 mm of the wild type and 9.9 ± 2.09 mm of shy2-2 root length. (F) arf19-1 and nph4-1/arf7 single mutants and arf19-1 nph4-1/arf7 double mutants exhibit auxin but not ethylene resistance. Roots of all mutants were significantly resistant to NAA (P < 0.05, ANOVA) but not to ACC (P > 0.05, ANOVA) in comparison to the control. 100% corresponds to 10.5 ± 1.24 mm of the wild type, 13.0 ± 1.28 mm of arf19-1, 11.5 ± 1.27 mm of nph4-1/arf7, and 11.0 ± 1.64 mm of arf19-1 nph4-1/arf7 root length. Seedlings were grown, if not specified differently, in the presence of 200 nM NAA and 1 μM ACC. Data are mean ±
sd
(except in [C]).
Figure 5.
Root growth of ethylene-insensitive mutants on auxin. (A) Root elongation of ethylene signaling mutants etr1-3 and ein2-1 is ACC resistant (P < 0.05, ANOVA). _etr1-3_ is wild-type-like sensitive to auxin (P > 0.05, ANOVA), and ein2-1 shows moderate, but statistically significant, auxin resistance (P < 0.05, ANOVA). 100% corresponds to 12.1 ± 2.53 mm of the wild type, 12.0 ± 2.75 mm of _etr1-3_, and 16.4 ± 0.89 mm of _ein2-1_ root length. **(B)** Root epidermal cell elongation of _etr1-3_ and _ein2-1_ is ACC resistant (P < 0.05, ANOVA). Root epidermal cells of _etr1-3_ are wild-type-like sensitive to auxin (P > 0.05, ANOVA), and ein2-1 shows moderate, but statistically significant, auxin resistance (P < 0.05, ANOVA). (C) Ectopic DR5-GUS expression was induced by auxin but not by ACC in etr1-3 and ein2-1 mutants. Insets: strong DR5-GUS staining in the differentiation zone after auxin treatment. Five-day-old seedlings were grown in the presence of 200 nM NAA and 1 μM ACC. Data are means ±
sd
.
Figure 6.
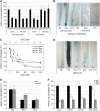
Local Stimulation of Auxin Biosynthesis by Ethylene. (A) ACC induces DR5 expression in roots of the wild type and eir1-1 but not that of the etr1-3 mutant (etr1-3/+ dominant phenotype, +/+ wild-type segregant). Intact seedlings (black bars) or detached roots (gray bars) were treated with or without 1 μM ACC. GUS activity in the roots was measured by a fluorimetric assay. (B) The 1-mm root tip segment comprises the root meristem and elongation zone. (C) ACC treatment increases auxin levels in the last 1 mm of the root tip of the wild type but not of the etr1-3 mutant. (D) AVG reduces the auxin biosynthesis rate in the root tip. By contrast, ACC treatment does not affect the auxin biosynthesis rate in the last 1 mm of the root tip. Results of one of three independent experiments are presented. Data are means ±
sd
.
Figure 7.
Ethylene-Modulated Expression of Auxin Transport Components. (A) ACC upregulates the PIN2 expression of proPIN2-GUS and proPIN2-PIN2:GFP. ACC effect is inhibited by silver ions. (B) ACC upregulates the AUX1 expression of proAUX1-GUS and proAUX1-AUX1:YFP. ACC effect is inhibited by silver ions. (C) Quantification of membrane-located GFP/YFP fluorescence by image analysis of confocal sections; expression of proPIN2-PIN2:GFP and proAUX1-AUX1:YFP is significantly upregulated after ethylene treatment (P < 0.05, ANOVA). (D) qRT-PCR analysis did not reveal ethylene stimulatory effect on AUX1 and PIN2 expression in the last 2 mm of the root tip. Results of one of three independent experiments are presented. (E) qRT-PCR shows that AUX1, but not PIN2 expression, is reduced in etr1-3 in the last 2 mm of the root tip. AUX1 RNA levels were significantly lower in the etr1-3 mutant (P < 0.05, ANOVA). Results of one of three independent experiments are presented. Five-day-old seedlings were grown in the presence of 1 μM ACC and 20 μM AgNO3. Data are means ±
sd
.
Figure 8.
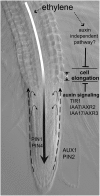
Model of Ethylene Action in the Root. Ethylene stimulates the biosynthesis of auxin that is transported toward the root tip. Subsequently, basipetally transported auxin activates the local auxin response that is regulated by the auxin receptor (TIR1) and AUX/IAA (AXR2/IAA7 and AXR3/IAA17) and inhibits the cell elongation. Increased expression of auxin efflux carriers (PIN1, PIN4, and PIN2) and influx carrier (AUX1) enhances the capacity of acropetal and basipetal auxin transport. This mechanism can account for most but not all ethylene effects on the root growth.
Similar articles
- Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation.
Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Swarup R, et al. Plant Cell. 2007 Jul;19(7):2186-96. doi: 10.1105/tpc.107.052100. Epub 2007 Jul 13. Plant Cell. 2007. PMID: 17630275 Free PMC article. - Cytokinin regulates root meristem activity via modulation of the polar auxin transport.
Ruzicka K, Simásková M, Duclercq J, Petrásek J, Zazímalová E, Simon S, Friml J, Van Montagu MC, Benková E. Ruzicka K, et al. Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4284-9. doi: 10.1073/pnas.0900060106. Epub 2009 Feb 25. Proc Natl Acad Sci U S A. 2009. PMID: 19246387 Free PMC article. - Auxin Controlled by Ethylene Steers Root Development.
Qin H, Huang R. Qin H, et al. Int J Mol Sci. 2018 Nov 20;19(11):3656. doi: 10.3390/ijms19113656. Int J Mol Sci. 2018. PMID: 30463285 Free PMC article. Review. - Copper regulates primary root elongation through PIN1-mediated auxin redistribution.
Yuan HM, Xu HH, Liu WC, Lu YT. Yuan HM, et al. Plant Cell Physiol. 2013 May;54(5):766-78. doi: 10.1093/pcp/pct030. Epub 2013 Feb 8. Plant Cell Physiol. 2013. PMID: 23396597 - Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk.
Hu Y, Vandenbussche F, Van Der Straeten D. Hu Y, et al. Planta. 2017 Mar;245(3):467-489. doi: 10.1007/s00425-017-2651-6. Epub 2017 Feb 10. Planta. 2017. PMID: 28188422 Review.
Cited by
- The root as a drill: an ethylene-auxin interaction facilitates root penetration in soil.
Santisree P, Nongmaithem S, Sreelakshmi Y, Ivanchenko M, Sharma R. Santisree P, et al. Plant Signal Behav. 2012 Feb;7(2):151-6. doi: 10.4161/psb.18936. Epub 2012 Feb 1. Plant Signal Behav. 2012. PMID: 22415043 Free PMC article. Review. - The karrikin signaling regulator SMAX1 controls Lotus japonicus root and root hair development by suppressing ethylene biosynthesis.
Carbonnel S, Das D, Varshney K, Kolodziej MC, Villaécija-Aguilar JA, Gutjahr C. Carbonnel S, et al. Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21757-21765. doi: 10.1073/pnas.2006111117. Epub 2020 Aug 17. Proc Natl Acad Sci U S A. 2020. PMID: 32817510 Free PMC article. - Asymmetric gibberellin signaling regulates vacuolar trafficking of PIN auxin transporters during root gravitropism.
Löfke C, Zwiewka M, Heilmann I, Van Montagu MC, Teichmann T, Friml J. Löfke C, et al. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3627-32. doi: 10.1073/pnas.1300107110. Epub 2013 Feb 7. Proc Natl Acad Sci U S A. 2013. PMID: 23391733 Free PMC article. - Physiological regulation and functional significance of shade avoidance responses to neighbors.
Keuskamp DH, Sasidharan R, Pierik R. Keuskamp DH, et al. Plant Signal Behav. 2010 Jun;5(6):655-62. doi: 10.4161/psb.5.6.11401. Epub 2010 Jun 1. Plant Signal Behav. 2010. PMID: 20404496 Free PMC article. Review.
References
- Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wirniewska, J., Moulinier-Anzola, J.C., Sieberer, T., Friml, J., and Luschnig, C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8 249–256. - PubMed
- Abel, S., Nguyen, M.D., Chow, W., and Theologis, A. (1995). ACS4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana: Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J. Biol. Chem. 270: 19093–19099. Erratum. J. Biol. Chem. 270 26020. - PubMed
- Benkova, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jürgens, G., and Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602. - PubMed
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schultz, B., and Feldmann, K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273 948–950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources