Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation - PubMed (original) (raw)
Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation
Ranjan Swarup et al. Plant Cell. 2007 Jul.
Abstract
Ethylene represents an important regulatory signal for root development. Genetic studies in Arabidopsis thaliana have demonstrated that ethylene inhibition of root growth involves another hormone signal, auxin. This study investigated why auxin was required by ethylene to regulate root growth. We initially observed that ethylene positively controls auxin biosynthesis in the root apex. We subsequently demonstrated that ethylene-regulated root growth is dependent on (1) the transport of auxin from the root apex via the lateral root cap and (2) auxin responses occurring in multiple elongation zone tissues. Detailed growth studies revealed that the ability of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid to inhibit root cell elongation was significantly enhanced in the presence of auxin. We conclude that by upregulating auxin biosynthesis, ethylene facilitates its ability to inhibit root cell expansion.
Figures
Figure 1.
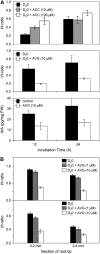
Manipulating Ethylene Synthesis in Arabidopsis Seedlings Also Alters the Synthesis and Abundance of Auxin. (A) IAA synthesis and abundance was measured in 6-d-old Arabidopsis seedlings that were incubated for 12 and 24 h with D2O medium containing the ethylene precursor ACC (top panel) or the ethylene synthesis inhibitor AVG (middle panel). IAA abundance was measured in 6-d-old Arabidopsis seedlings that were incubated for 12 and 24 h with D2O medium containing AVG (bottom panel). FW, fresh weight. (B) IAA synthesis was measured in 6-d-old Arabidopsis seedling roots treated with the ethylene synthesis inhibitor AVG. After 6 d of growth, either whole seedlings (top panel) or excised roots (bottom panel) were fed with D2O medium containing AVG for 24 h. IAA synthesis was measured in 2-mm segments of wild-type Arabidopsis roots sampled from tissues 0 to 2 mm and 2 to 4 mm from the apex. After correction for natural isotope abundances, ratios between the labeled tracer (mass-to-charge ratio [m/z] of 203, 204, and 205) and unlabeled tracee (m/z 202) were calculated for IAA (expressed as the t/t-ratio).
Figure 2.
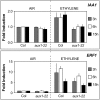
aux1 Blocks the Induction of Auxin (but Not Ethylene) Responsive Gene Expression after Ethylene Treatment. Transcript abundance of the auxin-responsive gene IAA1 (Abel and Theologis, 1995) and ethylene-responsive gene ERF1 (Solano et al., 1998) was measured employing qRT-PCR. RNA was extracted from 10-d-old wild-type and aux1-22 seedling roots 3, 6, and 15 h after treatment with ethylene (2 ppm) or ethylene-free air (controls). Data represent the average of three biological repeats (±
se
). All values are normalized to that of Col-0 exposed to ethylene-free air for the same time period.
Figure 3.
Induction of the Auxin-Responsive Reporter IAA2pro:GUS by Ethylene Is Dependent on EIN2 and AUX1. The expression of the IAA2pro:GUS reporter was monitored in the wild type ([A] and [F]), in the constitutive ethylene response mutant ctr1 ([B] and [G]), in the ethylene-insensitive mutant ein2 ([C] and [H]), in the auxin influx carrier null allele aux1-22 ([D] and [I]), and in the partial loss-of-function allele aux1-2 ([E] and [J]). Seedlings were either grown in air ([A] to [E]) or in the presence of 10 μL/L of ethylene ([F] to [J]).
Figure 4.
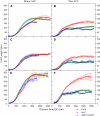
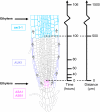
Inhibition of Root Cell Elongation by ACC Is Dependent on AUX1. Profiles of cortical ([A] and [B]), epidermal trichoblast ([C] and [D]), and atrichoblast ([E] and [F]) cell expansion in wild-type (circles), aux1 (diamonds), and aux1 J0951≫AUX1 (triangles) roots. Seedlings were either grown in the absence ([A], [C], and [E]) or in the presence ([B], [D], and [F]) of 1 μM ACC. Cell lengths were measured relative to the quiescent center (QC) at the root apex using confocal microscopy (see Methods).
Figure 5.
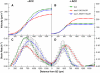
Kinematic Analysis of Root Growth Reveals That ACC Inhibits Rapid Cell Expansion. Profiles of velocity ([A] and [B]) and relative rates of cell expansion ([C] and [D]) along the root elongation zones of the wild type (circles), aux1 (diamonds), and aux1 J0951≫AUX1 (triangles) were obtained from kinematic analysis. Seedlings were grown either in the absence ([A] and [C]) or in the presence ([B] and [D]) of 1 μM ACC.
Figure 6.
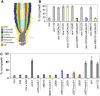
Ethylene-Inhibited Root Growth Is Dependent on Auxin Transport and Responses. (A) Schematic diagram summarizing Arabidopsis root apical tissue organization. LRC, lateral root cap; QC, quiescent center. (B) The sensitivity of aux1 root growth toward ethylene could be restored by targeting the expression of AUX1 in elongating epidermal and/or the lateral root cap cells in the aux1 J0951≫AUX1, aux1 Q1220≫AUX1, and aux1 M0013≫AUX1 lines, respectively. (C) The sensitivity of wild-type root growth toward ethylene could be partially disrupted by targeting the expression of axr3-1 in cortical/endodermal or epidermal cells in the J0951≫axr3-1 and J0571≫axr3-1 lines, respectively. Strong ethylene resistance was observed in the J0631≫axr3-1 line, which expresses axr3-1 in every elongation zone tissue. Seedlings were grown vertically on Murashige and Skoog (MS) plates either in air or ethylene (10 μL/L) for 5 d. Root growth in the presence or absence of ethylene was measured and expressed as a percentage of root growth compared with the air control. Asterisks indicate significant difference from the control (P < 0.05; see Supplemental Figure 5 online for further details). The color coding of bars either refers to the root tissues in which either AUX1 or axr3-1 expression is targeted or, if black, denotes them as controls. The aux1-22, etr1-1, and axr3-1 mutants represent ethylene-resistant controls.
Figure 7.
Induction of IAA2pro:GUS by Ethylene Is Blocked by axr2-1 and axr3-1. The expression of the IAA2pro:GUS reporter was monitored in a variety of auxin response mutant backgrounds either grown in air ([A] to [E]) or after ethylene treatment (10 μL/L; [F] to [J]).
Figure 8.
Model for Auxin Action during Ethylene-Regulated Root Growth. The model provides a spatial framework on which to place auxin components required for ethylene responses in roots. The schematic diagram of the Arabidopsis root apex is overlaid with the sites of action of auxin biosynthesis (WEI2/ASA1 and WEI7/ASB1) and transport (AUX1) components required for ethylene inhibition of root growth. Small arrows denote the basipetal flow of auxin from the root apex via the lateral root cap to the elongation zone. Sites of ethylene-responsive gene expression are denoted by large horizontal arrows. We also highlight that the dominant gain-of-function mutation axr3-1/iaa17 can disrupt ethylene inhibition of root growth in elongation zone tissues. Vertical arrows either represent the time required or distance moved by root cells as they progress through meristematic and elongation zones.
Similar articles
- Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings.
Camacho-Cristóbal JJ, Martín-Rejano EM, Herrera-Rodríguez MB, Navarro-Gochicoa MT, Rexach J, González-Fontes A. Camacho-Cristóbal JJ, et al. J Exp Bot. 2015 Jul;66(13):3831-40. doi: 10.1093/jxb/erv186. Epub 2015 Apr 28. J Exp Bot. 2015. PMID: 25922480 Free PMC article. - TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis.
Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z. Yang ZB, et al. Plant Cell. 2014 Jul;26(7):2889-904. doi: 10.1105/tpc.114.127993. Epub 2014 Jul 22. Plant Cell. 2014. PMID: 25052716 Free PMC article. - Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks.
Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BS, Muday GK. Lewis DR, et al. Plant Physiol. 2011 May;156(1):144-64. doi: 10.1104/pp.111.172502. Epub 2011 Mar 22. Plant Physiol. 2011. PMID: 21427279 Free PMC article. - Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk.
Hu Y, Vandenbussche F, Van Der Straeten D. Hu Y, et al. Planta. 2017 Mar;245(3):467-489. doi: 10.1007/s00425-017-2651-6. Epub 2017 Feb 10. Planta. 2017. PMID: 28188422 Review. - Deciphering Auxin-Ethylene Crosstalk at a Systems Level.
Zemlyanskaya EV, Omelyanchuk NA, Ubogoeva EV, Mironova VV. Zemlyanskaya EV, et al. Int J Mol Sci. 2018 Dec 14;19(12):4060. doi: 10.3390/ijms19124060. Int J Mol Sci. 2018. PMID: 30558241 Free PMC article. Review.
Cited by
- Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings.
Camacho-Cristóbal JJ, Martín-Rejano EM, Herrera-Rodríguez MB, Navarro-Gochicoa MT, Rexach J, González-Fontes A. Camacho-Cristóbal JJ, et al. J Exp Bot. 2015 Jul;66(13):3831-40. doi: 10.1093/jxb/erv186. Epub 2015 Apr 28. J Exp Bot. 2015. PMID: 25922480 Free PMC article. - Identification, characterization of an AP2/ERF transcription factor that promotes adventitious, lateral root formation in Populus.
Trupiano D, Yordanov Y, Regan S, Meilan R, Tschaplinski T, Scippa GS, Busov V. Trupiano D, et al. Planta. 2013 Aug;238(2):271-82. doi: 10.1007/s00425-013-1890-4. Epub 2013 May 5. Planta. 2013. PMID: 23645259 - A Model of Differential Growth-Guided Apical Hook Formation in Plants.
Žádníková P, Wabnik K, Abuzeineh A, Gallemi M, Van Der Straeten D, Smith RS, Inzé D, Friml J, Prusinkiewicz P, Benková E. Žádníková P, et al. Plant Cell. 2016 Oct;28(10):2464-2477. doi: 10.1105/tpc.15.00569. Epub 2016 Oct 17. Plant Cell. 2016. PMID: 27754878 Free PMC article. - A Complex Molecular Interplay of Auxin and Ethylene Signaling Pathways Is Involved in Arabidopsis Growth Promotion by Burkholderia phytofirmans PsJN.
Poupin MJ, Greve M, Carmona V, Pinedo I. Poupin MJ, et al. Front Plant Sci. 2016 Apr 12;7:492. doi: 10.3389/fpls.2016.00492. eCollection 2016. Front Plant Sci. 2016. PMID: 27148317 Free PMC article. - Identification of the primary lesion of toxic aluminum in plant roots.
Kopittke PM, Moore KL, Lombi E, Gianoncelli A, Ferguson BJ, Blamey FP, Menzies NW, Nicholson TM, McKenna BA, Wang P, Gresshoff PM, Kourousias G, Webb RI, Green K, Tollenaere A. Kopittke PM, et al. Plant Physiol. 2015 Apr;167(4):1402-11. doi: 10.1104/pp.114.253229. Epub 2015 Feb 10. Plant Physiol. 2015. PMID: 25670815 Free PMC article.
References
- Abel, S., and Theologis, A. (1995). A polymorphic bipartite motif signals nuclear targeting of early auxin-inducible proteins related to Ps-IAA4 from pea (Pisum sativum). Plant J. 8 87–96. - PubMed
- Alonso, J.M., and Stepanova, A.N. (2004). The ethylene signaling pathway. Science 306 1513–1515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous