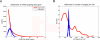Global and local architecture of the mammalian microRNA-transcription factor regulatory network - PubMed (original) (raw)
Global and local architecture of the mammalian microRNA-transcription factor regulatory network
Reut Shalgi et al. PLoS Comput Biol. 2007 Jul.
Abstract
microRNAs (miRs) are small RNAs that regulate gene expression at the posttranscriptional level. It is anticipated that, in combination with transcription factors (TFs), they span a regulatory network that controls thousands of mammalian genes. Here we set out to uncover local and global architectural features of the mammalian miR regulatory network. Using evolutionarily conserved potential binding sites of miRs in human targets, and conserved binding sites of TFs in promoters, we uncovered two regulation networks. The first depicts combinatorial interactions between pairs of miRs with many shared targets. The network reveals several levels of hierarchy, whereby a few miRs interact with many other lowly connected miR partners. We revealed hundreds of "target hubs" genes, each potentially subject to massive regulation by dozens of miRs. Interestingly, many of these target hub genes are transcription regulators and they are often related to various developmental processes. The second network consists of miR-TF pairs that coregulate large sets of common targets. We discovered that the network consists of several recurring motifs. Most notably, in a significant fraction of the miR-TF coregulators the TF appears to regulate the miR, or to be regulated by the miR, forming a diversity of feed-forward loops. Together these findings provide new insights on the architecture of the combined transcriptional-post transcriptional regulatory network.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
Figure 1. miRs and Target Genes in the TargetScan Dataset
(A) Distribution of the number of different miRs regulating each target gene in the TargetScan dataset. The thick red line represents the distribution in the original datasets, while each of the thin blue lines represents the distribution in one of the column-randomized matrices. The matrix contains only genes with at least one predicted site in their 3′ UTR. In each randomization, we shuffled the assignment of miRs to their targets, keeping constant the number of targets per miR. (B) Distribution of number of targets per miR in the TargetScan dataset. In the thick red line we depicted the original distribution, while each blue thin line represents the distribution in one of the 100 row-randomized matrices, which preserve the distribution of number of miRs targeting each gene.
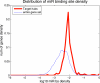
Figure 2. Distribution of the density of miRs in the 3′ UTRs of target hubs (thick red line) and all the genes (thin blue line) in the TargetScan dataset (all genes included in this figures have at least one miR site predicted in their 3′ UTR). The log10 densities were binned into bins of 0.1, and relative frequencies were plotted. Same analysis for the PicTar dataset is in Figure S2.
Figure 3. miR Co-Occurrence Network in the TargetScan Dataset
(A) The TargetScan miR co-occurrence network, at FDR level of 0.05. A node represents a miR and an edge connects between pairs of miRs with significant rate of co-occurrence. The nodes in the figure are arranged from most highly connected on the top, to most lowly connected, on the bottom. For interactive viewing of the network, using Pajek (
http://vlado.fmf.uni-lj.si/pub/networks/pajek/
), see Datasets S1 and S2. (B) Degree distribution in the TargetScan miR combinatorial regulation network (co-occurring miR pairs that passed FDR of 0.05).
Figure 4. Network Designs in the miR–TF Coregulation Network
The figure depicts the analyzed network motifs in the TargetScan and PicTar dataset, and with the use of TF binding sites in RefSeq genes promoters of 10 kb for both networks, and 5 kb for the PicTar network. The figure depicts, for each network motif, its architecture, the number of times it appears in each of the networks, the _p_-value and z-score for its over-representation in the network (as described in Materials and Methods), the total number of RefSeq genes that are regulated by this type of network design, and an example. *For the first design, the coregulating miR–TF pair, we state the range of hypergeometric _p_-values of pairs that passed FDR and are considered significant, and in brackets the FDR _p_-value of these pairs using the randomization co-occurrence test. **In addition, z-scores for significant pairs were calculated based on the co-occurrence edge-swapping randomization model (see Materials and Methods).
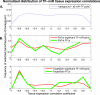
Figure 5. Tissue Expression Correlations between miRs and TFs
miR tissue expression in brain, liver, thymus, testes, and placenta were taken from [34]. mRNA tissue expression was taken from [35]. (A) Background distribution of all possible miR–TF pairs for which expression profiles can be derived. (B,C) Normalized histograms of correlation coefficients; the same distribution as in (A) was made, yet only for significantly co-occurring miR-TF pairs (red), and FFLs (green) in the PicTar (B) and TargetScan (C) networks. The figure shows the proportion of the various correlation coefficients divided by the background distribution depicted in (A).
Figure 6. Analysis of miR Clusters in the Human Genome
(A) Distribution of distances between all neighboring pre-miR genes in the human genome. (B) Distribution of tissue expression correlations between pairs of miRs: all possible pairs in the data (thin blue line) and pairs of miRs which reside in shared clusters (thick red line). In the inset are shown tissue expression correlations between pairs of miRs in the same genomic clusters versus distances between them. (C) Distribution of number of conserved TFBS 30 kb upstream of the 5′ most nucleotide in each miR clusters. Conserved TFBSs were taken from UCSC hg17.
Similar articles
- Transcriptome profiling of human hepatocytes treated with Aroclor 1254 reveals transcription factor regulatory networks and clusters of regulated genes.
Reymann S, Borlak J. Reymann S, et al. BMC Genomics. 2006 Aug 26;7:217. doi: 10.1186/1471-2164-7-217. BMC Genomics. 2006. PMID: 16934159 Free PMC article. - Analysis of regulatory network topology reveals functionally distinct classes of microRNAs.
Yu X, Lin J, Zack DJ, Mendell JT, Qian J. Yu X, et al. Nucleic Acids Res. 2008 Nov;36(20):6494-503. doi: 10.1093/nar/gkn712. Epub 2008 Oct 15. Nucleic Acids Res. 2008. PMID: 18927108 Free PMC article. - Topological patterns in microRNA-gene regulatory network: studies in colorectal and breast cancer.
Sengupta D, Bandyopadhyay S. Sengupta D, et al. Mol Biosyst. 2013 Jun;9(6):1360-71. doi: 10.1039/c3mb25518b. Epub 2013 Mar 11. Mol Biosyst. 2013. PMID: 23475160 - Transcription factor and microRNA co-regulatory loops: important regulatory motifs in biological processes and diseases.
Zhang HM, Kuang S, Xiong X, Gao T, Liu C, Guo AY. Zhang HM, et al. Brief Bioinform. 2015 Jan;16(1):45-58. doi: 10.1093/bib/bbt085. Epub 2013 Dec 4. Brief Bioinform. 2015. PMID: 24307685 Review. - Modeling microRNA-transcription factor networks in cancer.
Aguda BD. Aguda BD. Adv Exp Med Biol. 2013;774:149-67. doi: 10.1007/978-94-007-5590-1_9. Adv Exp Med Biol. 2013. PMID: 23377973 Review.
Cited by
- miRNA targeted signaling pathway in the early stage of denervated fast and slow muscle atrophy.
Li G, Li QS, Li WB, Wei J, Chang WK, Chen Z, Qiao HY, Jia YW, Tian JH, Liang BS. Li G, et al. Neural Regen Res. 2016 Aug;11(8):1293-303. doi: 10.4103/1673-5374.189195. Neural Regen Res. 2016. PMID: 27651778 Free PMC article. - Studying Dynamic Features in Myocardial Infarction Progression by Integrating miRNA-Transcription Factor Co-Regulatory Networks and Time-Series RNA Expression Data from Peripheral Blood Mononuclear Cells.
Shi H, Zhang G, Wang J, Wang Z, Liu X, Cheng L, Li W. Shi H, et al. PLoS One. 2016 Jul 1;11(7):e0158638. doi: 10.1371/journal.pone.0158638. eCollection 2016. PLoS One. 2016. PMID: 27367417 Free PMC article. - RNA-binding proteins regulate aldosterone homeostasis in human steroidogenic cells.
Fu R, Wellman K, Baldwin A, Rege J, Walters K, Hirsekorn A, Riemondy K, Rainey WE, Mukherjee N. Fu R, et al. RNA. 2021 Jun 1;27(8):933-45. doi: 10.1261/rna.078727.121. Online ahead of print. RNA. 2021. PMID: 34074709 Free PMC article. - Competing endogenous RNA database.
Sarver AL, Subramanian S. Sarver AL, et al. Bioinformation. 2012;8(15):731-3. doi: 10.6026/97320630008731. Epub 2012 Aug 3. Bioinformation. 2012. PMID: 23055620 Free PMC article. - Generation of Realistic Gene Regulatory Networks by Enriching for Feed-Forward Loops.
Zhivkoplias EK, Vavulov O, Hillerton T, Sonnhammer ELL. Zhivkoplias EK, et al. Front Genet. 2022 Feb 10;13:815692. doi: 10.3389/fgene.2022.815692. eCollection 2022. Front Genet. 2022. PMID: 35222536 Free PMC article.
References
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–70. - PubMed
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous