Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy - PubMed (original) (raw)
doi: 10.1186/1476-4598-6-48.
Jonathan E McDunn, Peter O Simon Jr, Peter S Goedegebuure, Jinbin Xu, Lynne Jones, Katherine Chang, Fabian Johnston, Kathryn Trinkaus, Richard S Hotchkiss, Robert H Mach, William G Hawkins
Affiliations
- PMID: 17631687
- PMCID: PMC1939854
- DOI: 10.1186/1476-4598-6-48
Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy
Hiroyuki Kashiwagi et al. Mol Cancer. 2007.
Abstract
Background: Resistance to modern adjuvant treatment is in part due to the failure of programmed cell death. Therefore the molecules that execute the apoptotic program are potential targets for the development of anti-cancer therapeutics. The sigma-2 receptor has been found to be over-expressed in some types of malignant tumors, and, recently, small molecule ligands to the sigma-2 receptor were found to induce cancer cell apoptosis.
Results: The sigma-2 receptor was expressed at high levels in both human and murine pancreas cancer cell lines, with minimal or limited expression in normal tissues, including: brain, kidney, liver, lung, pancreas and spleen. Micro-PET imaging was used to demonstrate that the sigma-2 receptor was preferentially expressed in tumor as opposed to normal tissues in pancreas tumor allograft-bearing mice. Two structurally distinct sigma-2 receptor ligands, SV119 and WC26, were found to induce apoptosis to mice and human pancreatic cancer cells in vitro and in vivo. Sigma-2 receptor ligands induced apoptosis in a dose dependent fashion in all pancreatic cell lines tested. At the highest dose tested (10 muM), all sigma-2 receptor ligands induced 10-20% apoptosis in all pancreatic cancer cell lines tested (p < 0.05). In pancreas tumor allograft-bearing mice, a single bolus dose of WC26 caused approximately 50% apoptosis in the tumor compared to no appreciable apoptosis in tumor-bearing, vehicle-injected control animals (p < 0.0001). WC26 significantly slowed tumor growth after a 5 day treatment compared to vehicle-injected control animals (p < 0.0001) and blood chemistry panels suggested that there is minimal peripheral toxicity.
Conclusion: We demonstrate a novel therapeutic strategy that induces a significant increase in pancreas cancer cell death. This strategy highlights a new potential target for the treatment of pancreas cancer, which has little in the way of effective treatments.
Figures
Figure 1
Structure of sigma ligands utilized in this paper. Panel A: Haloperidol is a non-specific sigma-1 and sigma-2 receptor ligand. Panel B: Pentazocine is a sigma-1 specific ligand. Panel C: Sigma-2 receptor-specific ligands SV119 and WC26. Panel D: Chemically-labeled sigma-2 receptor-specific ligands K05-138 (fluorescein-labeled), RHM-1 (3H-labeled), and RHM-4 (18F-labeled).
Figure 2
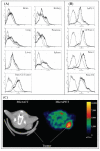
Sigma-2 ligands preferentially bind to cancer as opposed to normal tissues. (A): Single cell suspensions were prepared from liver, lung, pancreas, brain, kidney, spleen and pancreatic tumors (panc-02 bearing mice). Single cell suspensions were incubated for 1 hour with KO5-138 (a fluorescent labeled sigma-2 receptor ligand, at 50 nM (thin doted line) or 100 nM (thick solid line) of ligand, or left unstained (thin solid line). FACS histograms demonstrate 100 fold more florescence in tumors compared to normal tissues. (B): Human pancreatic cancer lines (CFPAC-1, Panc-1, AsPC-1) demonstrate similar high degree of sigma-2 ligand binding to when compared to our murine model (Panc-02). Fluorescein signal peaks were shown in unstained control (thin solid line), 50 nM of ligand (dotted line), or 100 nM of ligand (thick solid line). The experiment was repeated twice with identical results. (C): Representative Micro PET/CT images of pancreas adenocarcinoma (Panc-02) bearing C57BL/6 mice administered RHM-4 (18F-labled sigma-2 ligand). The tumor indicated by arrows was approximately 1 cm3. The additional hot spot represents metastatic tumor in a regional lymph node.
Figure 3
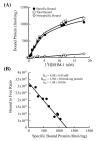
Scatchard analysis of [3H]RHM-1 binding to the sigma-2 receptors in membrane homogenates from Panc-02 tumor allografts. (A): Representative saturation binding experiments which show the total bound, non-specific bound and specific bound. (B): Representative Scatchard plots which were used to determine _K_d and _B_max values.
Figure 4
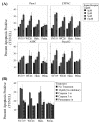
Sigma-2 receptor ligands induce apoptosis of pancreatic cancer cells via a caspase 3 dependent pathway. (A): Treatment with WC26, SV119 and haloperidol (Halo.) caused dose-dependent apoptosis in all pancreatic cancer cell lines tested. At the highest dose tested, all sigma-2 receptor ligands resulted in significant apoptosis (25–35%, p < 0.05). (B): Sigma-2 related apoptosis was prevented by pan-caspase inhibitor (Z-VAD-fmk) and caspase-3/7 inhibitor (DEVD-CHO), while the caspase-1 inhibitor (YVAD-CHO) had no effect (Panc-02 cells).
Figure 5
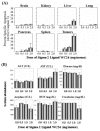
Systemic administration of Sigma-2 receptor ligand induces substantial tumor apoptosis without major toxicity. (A): Systemic administration of the sigma-2 receptor ligand WC26 induces considerable apoptosis in Panc-02 tumor allografts (up to 50% of tumor cells were active caspase-3 positive following a single 2 mg dose of WC26, p < 0.0001). Data reported as percent specific apoptosis (percent specific apoptosis = observed apoptosis – tissue specific background apoptosis). (B): The mice appeared normal and no apparent toxicity was noted in serum biochemical analysis or by immunohistochemistry (data not shown). Reference ranges for the blood chemistry panel in C57Bl/6 mice: ALT: 18–184 U/L, AST: 55–251 U/L, glucose: 174–335 mg/dL, amylase: 2595 +/- 212 U/L, BUN: 34–58 mg/dL, creatinine < 1.1 mg/dL.
Figure 6
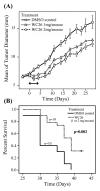
Systemic administration of sigma-2 receptor ligand slows tumor growth and improves survival. Mice with established pancreas tumors were treated with 5 days of WC26 in one of two dose ranges (2 mg/day, n = 9; Dash line or 1 mg/day n = 10; dashed line) or vehicle control (20% DMSO n = 10; solid line). (A): Mice treated with WC26 had smaller tumors compared to animals vehicle control (p < 0.0001). The two treatment groups were not statistically different. The double-headed arrow denotes the treatment period (5 days). **(B):** Survival of mice treated with WC26 compared favorably to the mice treated vehicle control (p= 0.002). Survival endpoints were defined as tumor diameter >15 mm or tumor ulceration.
Similar articles
- Targeted pancreatic cancer therapy with the small molecule drug conjugate SW IV-134.
Hashim YM, Spitzer D, Vangveravong S, Hornick MC, Garg G, Hornick JR, Goedegebuure P, Mach RH, Hawkins WG. Hashim YM, et al. Mol Oncol. 2014 Jul;8(5):956-67. doi: 10.1016/j.molonc.2014.03.005. Epub 2014 Mar 26. Mol Oncol. 2014. PMID: 24731702 Free PMC article. - Sigma-2 receptor ligands potentiate conventional chemotherapies and improve survival in models of pancreatic adenocarcinoma.
Kashiwagi H, McDunn JE, Simon PO Jr, Goedegebuure PS, Vangveravong S, Chang K, Hotchkiss RS, Mach RH, Hawkins WG. Kashiwagi H, et al. J Transl Med. 2009 Mar 26;7:24. doi: 10.1186/1479-5876-7-24. J Transl Med. 2009. PMID: 19323815 Free PMC article. - Sigma-2 receptor agonist derivatives of 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) induce cell death via mitochondrial superoxide production and caspase activation in pancreatic cancer.
Pati ML, Hornick JR, Niso M, Berardi F, Spitzer D, Abate C, Hawkins W. Pati ML, et al. BMC Cancer. 2017 Jan 13;17(1):51. doi: 10.1186/s12885-016-3040-4. BMC Cancer. 2017. PMID: 28086830 Free PMC article. - Potential applications for sigma receptor ligands in cancer diagnosis and therapy.
van Waarde A, Rybczynska AA, Ramakrishnan NK, Ishiwata K, Elsinga PH, Dierckx RA. van Waarde A, et al. Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2703-14. doi: 10.1016/j.bbamem.2014.08.022. Epub 2014 Aug 27. Biochim Biophys Acta. 2015. PMID: 25173780 Review. - Sigma receptors in oncology: therapeutic and diagnostic applications of sigma ligands.
van Waarde A, Rybczynska AA, Ramakrishnan N, Ishiwata K, Elsinga PH, Dierckx RA. van Waarde A, et al. Curr Pharm Des. 2010;16(31):3519-37. doi: 10.2174/138161210793563365. Curr Pharm Des. 2010. PMID: 21050178 Review.
Cited by
- Functional assays to define agonists and antagonists of the sigma-2 receptor.
Zeng C, Rothfuss JM, Zhang J, Vangveravong S, Chu W, Li S, Tu Z, Xu J, Mach RH. Zeng C, et al. Anal Biochem. 2014 Mar 1;448:68-74. doi: 10.1016/j.ab.2013.12.008. Epub 2013 Dec 12. Anal Biochem. 2014. PMID: 24333652 Free PMC article. - Multifunctional thiosemicarbazones and deconstructed analogues as a strategy to study the involvement of metal chelation, Sigma-2 (σ2) receptor and P-gp protein in the cytotoxic action: In vitro and in vivo activity in pancreatic tumors.
Pati ML, Niso M, Spitzer D, Berardi F, Contino M, Riganti C, Hawkins WG, Abate C. Pati ML, et al. Eur J Med Chem. 2018 Jan 20;144:359-371. doi: 10.1016/j.ejmech.2017.12.024. Epub 2017 Dec 8. Eur J Med Chem. 2018. PMID: 29287249 Free PMC article. - Therapeutic targeting of pancreatic cancer utilizing sigma-2 ligands.
Hornick JR, Spitzer D, Goedegebuure P, Mach RH, Hawkins WG. Hornick JR, et al. Surgery. 2012 Sep;152(3 Suppl 1):S152-6. doi: 10.1016/j.surg.2012.05.014. Epub 2012 Jul 3. Surgery. 2012. PMID: 22763259 Free PMC article. Review. - Characterization of spherulites as a lipidic carrier for low and high molecular weight agents.
Zhang P, Huang Y, Makhov AM, Gao X, Zhang P, Li S. Zhang P, et al. Pharm Res. 2013 Jun;30(6):1525-35. doi: 10.1007/s11095-013-0990-y. Epub 2013 Apr 12. Pharm Res. 2013. PMID: 23579481 Free PMC article. - Fluorescent derivatives of σ receptor ligand 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) as a tool for uptake and cellular localization studies in pancreatic tumor cells.
Abate C, Hornick JR, Spitzer D, Hawkins WG, Niso M, Perrone R, Berardi F. Abate C, et al. J Med Chem. 2011 Aug 25;54(16):5858-67. doi: 10.1021/jm200591t. Epub 2011 Jul 20. J Med Chem. 2011. PMID: 21744858 Free PMC article.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
- Prochazkova J, Lichnovsky V, Kylarova D, Erdosova B, Vranka P. Involvement of p53 and Bcl-2 family proteins in regulating programmed cell death and proliferation in human embryogenesis. Gen Physiol Biophys. 2004;23:209–229. - PubMed
- Bergman PJ, Harris D. Radioresistance, chemoresistance, and apoptosis resistance. The past, present, and future. Vet Clin North Am Small Anim Pract. 1997;27:47–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM044118/GM/NIGMS NIH HHS/United States
- GM055194/GM/NIGMS NIH HHS/United States
- GM044118/GM/NIGMS NIH HHS/United States
- 5P30 DK052574/DK/NIDDK NIH HHS/United States
- R01 GM055194/GM/NIGMS NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- R37 GM044118/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical