The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2 - PubMed (original) (raw)
The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2
Wenhui Li et al. Virology. 2007.
Abstract
The cellular receptor for human coronavirus NL63 (HCoV-NL63), a group I coronavirus, is angiotensin-converting enzyme2 (ACE2). ACE2 is also the receptor for the SARS coronavirus (SARS-CoV), a group II coronavirus. Here we describe the ability of HCoV-NL63 to utilize a number of ACE2 variants previously characterized as SARS-CoV receptors. Several ACE2 variants that reduced SARS-CoV S-protein association similarly reduced that of HCoV-NL63, whereas alteration of a number of solvent-exposed ACE2 residues did not interfere with binding by either S protein. One notable exception is ACE2 residue 354, at the boundary of the SARS-CoV binding site, whose alteration markedly inhibited utilization by the HCoV-NL63 but not SARS-CoV S proteins. In addition, the SARS-CoV S-protein receptor-binding domain inhibited entry mediated by the HCoV-NL63 S protein. These studies indicate that HCoV-NL63, like SARS-CoV, associates region of human ACE2 that includes a key loop formed by beta-strands 4 and 5.
Figures
Fig. 1
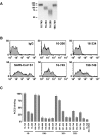
Residues 301 through 643 of HCoV-NL63 spike protein contribute to ACE2 association. (A) Fusion proteins expressing the indicated fragments of the HCoV-NL63 S protein and the Fc region of human IgG1 were purified from transfected HEK293T cells. Expression of S1-Ig (residues 16–749) and smaller variants were normalized for expression by Coomassie staining. (B) 5 μg/ml of human IgG, HCoV-NL63 S1-Ig (residues 16–749), or the indicated truncation variants thereof, or SARS-CoV S1-Ig was incubated with human-ACE2-expressing HEK293T cells. Cells were washed and ACE2 association analyzed by flow cytometry. Mean fluorescence intensity: IgG, 3.4; 16–200, 3.7; 16–334, 4.4; SARS-CoV S1, 79.3; 16–749, 18.2; 198–749, 20.5. (C) Experiment similar to that in panel B except that a wider range of truncation variants of HCoV-NL63 S1-Ig were assayed. Binding, measured as mean fluorescence intensity, is shown as percentage of binding observed with HCoV-NL63 S1-Ig.
Fig. 2
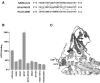
Alteration of a putative HCoV-NL63 receptor-binding motif modulates ACE2 association. (A) Alignment of the SARS-CoV RBM with similar regions of HCoV-NL63 and HCoV-229E S proteins. SARS-CoV residues that directly contact ACE2 are highlighted, as is HCoV-NL63 S-protein asparagine 578, whose alteration to tyrosine enhances association with ACE2. HCoV-NL63 residues whose alteration decreases association with ACE2 are underlined. (B) Experiment similar to that of Fig. 1C except that HCoV-NL63 S1-Ig(301-749) variants altered in its putative receptor-binding motif are characterized. (C) The structure of the SARS-CoV RBD bound to ACE2 (white). The RBM is shown in light grey, and RBM residues in direct contact with ACE2 are shown in black. Disulfides forming a small loop, which may be similar to loops in HCoV-NL63 and HCoV-229E, are indicated. Tyrosine 475, adjacent to the C-terminal cysteine of this loop, is represented as spheres.
Fig. 3
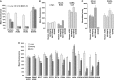
HCoV-NL63 and SARS-CoV S proteins bind similar sites on human ACE2. (A) HEK293T cells expressing N-terminally tagged forms of human, rat, or two variants of palm civet ACE2 proteins incubated with anti-tag antibody, or HCoV-NL63 S1-Ig(16–749) or S1-Ig(301–749). Cells were washed and analyzed by flow cytometry. Results are indicated as the percentage of mean fluorescence intensity observed with cells expressing human ACE2 and incubated with anti-tag antibody. (B) An experiment similar to (A) except that human ACE2, and three indicated variants thereof, was analyzed for binding to anti-tag antibody, HCoV-NL63 S1-Ig(301–749), and SARS-CoV S1-Ig. (C) Lentiviruses expressing luciferase and pseudotyped with the S proteins of HCoV-NL63 or SARS-CoV were incubated with cells transfected with human ACE2 or the indicated human ACE2 variants, as previously described (Li et al., 2005c, Sui et al., 2005). Entry, as measured by luciferase activity, is shown as percentage observed for wild-type human ACE2. (D) Experiments similar to those of (B) and (C) except that human ACE2 variants proximal to the SARS-CoV binding site are characterized for expression, HCoV-NL63 S1-Ig(301–749) binding, and HCoV-NL63 S-protein-mediated infection. See Supplemental data for results of experiments characterizing variants that do not contact the SARS-CoV RBD.
Fig. 4
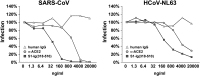
The SARS-CoV RBD inhibits HCoV-NL63 S-protein-mediated infection. Lentiviruses expressing luciferase and pseudotyped with the S proteins of SARS-CoV or HCoV-NL63 were incubated with ACE2-expressing HEK293T cells in the presence of the indicated concentrations of affinity purified goat anti-ACE2 antibody (Li et al., 2003), SARS-CoV RBD-Ig, or human IgG. Infection is expressed as a percentage of luciferase activity observed in the absence of inhibitor.
Fig. 5
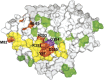
ACE2 residues critical to HCoV-NL63 S1-Ig association. A “virus-eye” view of the human ACE2 surface is shown. Residues in contact with the SARS-CoV RBD are indicated in yellow and orange. Residues whose alteration affects HCoV-CoV S1 binding were colored orange. Glycine 354, whose alteration to aspartic acid abolishes HCoV-NL63 binding, but not that of SARS-CoV, is shown in purple. Residues whose alteration does not alter ACE2 association with either S protein are shown in green. Some green residues listed in Supplementary Table 1 are not visible in this view.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous