Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms - PubMed (original) (raw)
Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms
Marlen Knobloch et al. J Neurosci. 2007.
Abstract
Amyloid beta (Abeta) oligomers are derived from proteolytic cleavage of amyloid precursor protein (APP) and can impair memory and hippocampal long-term potentiation (LTP) in vivo and in vitro. They are recognized as the primary neurotoxic agents in Alzheimer's disease. The mechanisms underlying such toxicity on synaptic functions are complex and not fully understood. Here, we provide the first evidence that these mechanisms involve protein phosphatase 1 (PP1). Using a novel transgenic mouse model expressing human APP with the Swedish and Arctic mutations that render Abeta more prone to form oligomers (arcAbeta mice), we show that the LTP impairment induced by Abeta oligomers can be fully reversed by PP1 inhibition in vitro. We further demonstrate that the genetic inhibition of endogenous PP1 in vivo confers resistance to Abeta oligomer-mediated toxicity and preserves LTP. Overall, these results reveal that PP1 is a key player in the mechanisms of AD pathology.
Figures
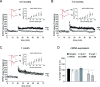
Figure 1.
CA1 hippocampal LTP is impaired in arcAβ mice. A, B, CA1 hippocampal LTP is severely impaired in slices from 3.5- and 7.5-month-old arcAβ mice (n = 5 tg, 5 wt mice) but basal transmission is normal (right inset). C, LTP and basal transmission (right inset) are normal in slices from 1-month-old arcAβ mice (n = 5 tg, 5 wt mice). Left insets, Individual fEPSP traces for tg and wt mice before (black) and after (red) LTP induction. Calibration: A, B, 0.4 mV, 5 ms; C, 0.6 mV, 5 ms. D, Real-time RT-PCR: normal mRNA expression of synaptophysin, NMDA and AMPA receptors, and CaMKII, but reduced arg3.1 (67%; range, 58–78%; n = 5 tg and 5 wt mice) and zif268 (52%; range, 45–61%) expression in the hippocampus of 6-month-old arcAβ mice. Error bars represent SEM. *p < 0.05. norm., Normalized; Synapto., synaptophysin; Tg, transgenic.
Figure 2.
In vitro inhibition of PP1 reverses Aβ oligomer-mediated LTP impairment. A, B, PP1 is inhibited by bath application of 1 n
m
tautomycin (tauto) in slices from 3-month-old arcAβ mice and wild-type littermates. Tautomycin fully reverses the LTP deficit in slices from tg mice (n = 5) but has no effect in wt (n = 5 mice). It also does not change basal transmission (right insets). Left insets, Individual fEPSP traces for tg and wt mice before (black) and after (red) LTP induction. Calibration: 0.3 mV, 5 ms. C, Phosphatase activity assays showing that 1 n
m
tautomycin specifically inhibits the activity of recombinant PP1 but not of recombinant calcineurin. D, Frequency–response curve showing mean fEPSP slopes over 10 min starting 35 min after stimulation with 1, 2, 5, 10, and 100 Hz in slices from tg and wt mice treated or not with tautomycin (n = 3–5 slices per condition and frequency). E, PP1 inhibition with 1 n
m
tautomycin also reverses the LTP deficit seen in 3-month-old APPSwe/PS1 mice, another mouse model of AD (n = 5 tg). Error bars represent SEM. CN, Calcineurin; Tg, transgenic; w/o, without.
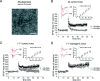
Figure 3.
Endogenous PP1 inhibition in vivo confers resistance to Aβ oligomer-mediated toxicity. A, Electron microscopy of Aβ oligomer preparation produced from synthetic Aβ1–42 (according to Klein, 2002) showing globular Aβ assemblies of 7–12 nm after 24 h incubation at 4°C. Scale bar, 200 nm. B, Confirmation of Aβ oligomer-mediated toxicity in wt mice. Aβ oligomers were bath applied to wt slices for at least 1 h. Aβ oligomers impair LTP (n = 3–4; 3 slices with Aβ oligomers and 4 control slices). Right inset, Basal transmission was not affected. Left insets, Individual fEPSP traces with and without Aβ oligomer application before (black) and after (red) LTP induction. Calibration: 0.6 mV, 5 ms. C, D, Aβ oligomers are bath applied for at least 1 h to slices from I-1* transgenic mice and control littermates. Aβ oligomers impair LTP in I-1* control mice (n = 5) but not in I-1* transgenic mice (n = 5). Right inset, Basal transmission is not affected. Left insets, Individual fEPSP traces with and without application of Aβ oligomers before (black) and after (red) LTP induction. Calibration: 0.6 mV, 5 ms. Error bars represent SEM. oligo., Oligomer.
Similar articles
- Pyruvate prevents the inhibition of the long-term potentiation induced by amyloid-β through protein phosphatase 2A inactivation.
Wang X, Takata T, Bai X, Ou F, Yokono K, Sakurai T. Wang X, et al. J Alzheimers Dis. 2012;30(3):665-73. doi: 10.3233/JAD-2012-101869. J Alzheimers Dis. 2012. PMID: 22451307 - Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors.
Shemer I, Holmgren C, Min R, Fülöp L, Zilberter M, Sousa KM, Farkas T, Härtig W, Penke B, Burnashev N, Tanila H, Zilberter Y, Harkany T. Shemer I, et al. Eur J Neurosci. 2006 Apr;23(8):2035-47. doi: 10.1111/j.1460-9568.2006.04733.x. Eur J Neurosci. 2006. PMID: 16630051 - WASP-1, a canonical Wnt signaling potentiator, rescues hippocampal synaptic impairments induced by Aβ oligomers.
Vargas JY, Ahumada J, Arrázola MS, Fuenzalida M, Inestrosa NC. Vargas JY, et al. Exp Neurol. 2015 Feb;264:14-25. doi: 10.1016/j.expneurol.2014.11.005. Epub 2014 Nov 20. Exp Neurol. 2015. PMID: 25450465 - Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice.
Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Trinchese F, et al. Ann Neurol. 2004 Jun;55(6):801-14. doi: 10.1002/ana.20101. Ann Neurol. 2004. PMID: 15174014
Cited by
- Impact of social relationships on Alzheimer's memory impairment: mechanistic studies.
Hsiao YH, Chang CH, Gean PW. Hsiao YH, et al. J Biomed Sci. 2018 Jan 11;25(1):3. doi: 10.1186/s12929-018-0404-x. J Biomed Sci. 2018. PMID: 29325565 Free PMC article. Review. - The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death.
Söllvander S, Nikitidou E, Gallasch L, Zyśk M, Söderberg L, Sehlin D, Lannfelt L, Erlandsson A. Söllvander S, et al. J Neuroinflammation. 2018 Mar 28;15(1):98. doi: 10.1186/s12974-018-1134-4. J Neuroinflammation. 2018. PMID: 29592816 Free PMC article. - Alzheimer's-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal.
Pitt J, Roth W, Lacor P, Smith AB 3rd, Blankenship M, Velasco P, De Felice F, Breslin P, Klein WL. Pitt J, et al. Toxicol Appl Pharmacol. 2009 Oct 15;240(2):189-97. doi: 10.1016/j.taap.2009.07.018. Epub 2009 Jul 23. Toxicol Appl Pharmacol. 2009. PMID: 19631677 Free PMC article. - Cathepsin B Improves ß-Amyloidosis and Learning and Memory in Models of Alzheimer's Disease.
Embury CM, Dyavarshetty B, Lu Y, Wiederin JL, Ciborowski P, Gendelman HE, Kiyota T. Embury CM, et al. J Neuroimmune Pharmacol. 2017 Jun;12(2):340-352. doi: 10.1007/s11481-016-9721-6. Epub 2016 Dec 13. J Neuroimmune Pharmacol. 2017. PMID: 27966067 Free PMC article. - Parishin C's prevention of Aβ 1-42-induced inhibition of long-term potentiation is related to NMDA receptors.
Liu Z, Wang W, Feng N, Wang L, Shi J, Wang X. Liu Z, et al. Acta Pharm Sin B. 2016 May;6(3):189-97. doi: 10.1016/j.apsb.2016.03.009. Epub 2016 Apr 22. Acta Pharm Sin B. 2016. PMID: 27175329 Free PMC article.
References
- Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. - PubMed
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. - PubMed
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. - PubMed
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources