Proteolipid protein is required for transport of sirtuin 2 into CNS myelin - PubMed (original) (raw)
. 2007 Jul 18;27(29):7717-30.
doi: 10.1523/JNEUROSCI.1254-07.2007.
Katja Kuhlmann, Siming Shen, Marina Uecker, Anke Schardt, Kalina Dimova, Foteini Orfaniotou, Ajit Dhaunchak, Bastian G Brinkmann, Wiebke Möbius, Lenny Guarente, Patrizia Casaccia-Bonnefil, Olaf Jahn, Klaus-Armin Nave
Affiliations
- PMID: 17634366
- PMCID: PMC2676101
- DOI: 10.1523/JNEUROSCI.1254-07.2007
Proteolipid protein is required for transport of sirtuin 2 into CNS myelin
Hauke B Werner et al. J Neurosci. 2007.
Abstract
Mice lacking the expression of proteolipid protein (PLP)/DM20 in oligodendrocytes provide a genuine model for spastic paraplegia (SPG-2). Their axons are well myelinated but exhibit impaired axonal transport and progressive degeneration, which is difficult to attribute to the absence of a single myelin protein. We hypothesized that secondary molecular changes in PLP(null) myelin contribute to the loss of PLP/DM20-dependent neuroprotection and provide more insight into glia-axonal interactions in this disease model. By gel-based proteome analysis, we identified >160 proteins in purified myelin membranes, which allowed us to systematically monitor the CNS myelin proteome of adult PLP(null) mice, before the onset of disease. We identified three proteins of the septin family to be reduced in abundance, but the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase sirtuin 2 (SIRT2) was virtually absent. SIRT2 is expressed throughout the oligodendrocyte lineage, and immunoelectron microscopy revealed its association with myelin. Loss of SIRT2 in PLP(null) was posttranscriptional, suggesting that PLP/DM20 is required for its transport into the myelin compartment. Because normal SIRT2 activity is controlled by the NAD+/NADH ratio, its function may be coupled to the axo-glial metabolism and the long-term support of axons by oligodendrocytes.
Figures
Figure 1.
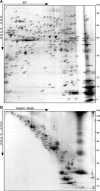
Gel-based myelin proteome maps. A, B, Myelin 2 from wild-type animals was two-dimensionally separated in different gel systems, and proteins visualized by colloidal Coomassie staining were identified by MALDI-TOF-MS. The spot numbers correspond to proteins listed in Table 1. A, 2D-IEF/SDS-PAGE with IEF in a nonlinear pH gradient (pH 3–10) as the first dimension and SDS-PAGE as the second dimension. B, 2D-16-BAC/SDS-PAGE with separation in a 16-BAC gel as the first and SDS-PAGE as the second dimension. Representative gels are shown.
Figure 2.
Comparison of myelin proteome libraries. A, Venn diagram comparing the number of myelin-associated proteins identified by MS after separation by 2D-IEF/SDS-PAGE in the present study and those reported previously based on the same technique (Taylor et al., 2004; Vanrobaeys et al., 2005). B , Comparison of myelin-associated proteins identified in this study by 2D-IEF/SDS-PAGE or 2D-16-BAC/SDS-PAGE. The classification corresponds to Table 1. Classification as mitochondrial or extracellular proteins was based on Swiss-Prot data entries. Note that known myelin proteins and proteins with a transmembrane (TM) domain were more efficiently identified in the 2D-16-BAC/SDS-PAGE approach. C, Venn diagram comparing the number of myelin-associated proteins identified in the present study by gel-based 2D-16-BAC/SDS-PAGE, and those previously reported based on gel-free LC/LC-MS (Vanrobaeys et al., 2005; Roth et al., 2006). D, Venn diagram comparing myelin proteome libraries by technical approaches. Numbers of proteins detected by 2D-IEF/SDS-PAGE (Taylor et al., 2004; Vanrobaeys et al., 2005; this study) were compared with gel-free LC/LC (Vanrobaeys et al., 2005; Roth et al., 2006) or 2D-16-BAC/SDS-PAGE (this study). Note the high overlap of proteins identified independent of the technique used.
Figure 3.
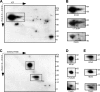
Differential myelin proteome analysis of the PLP_null_ mouse model of hereditary spastic paraplegia (SPG-2). A, 2D-DIGE of wild-type (WT; false-colored in blue) and PLP_null_ (false-colored in orange) myelin 2. Proteins less abundant in PLP_null_ myelin 2 constituted blue spots and were identified by MALDI-TOF-MS. B, Separation of detection channels and enlargement of the gel region marked by the red frame in A. Proteins associated with wild-type myelin (left; blue channel from A) included spots constituted by the septins SEPT2, SEPT4, and SEPT8 and four spots constituted by SIRT2 (I–IV, arrows). The principal spot pattern is similar in PLP_null_ myelin (right; orange channel from A), but spots constituted by SEPT2, SEPT4, and SEPT8 are diminished, and SIRT2 is virtually lacking (arrows). C, Relative intensities of spots constituted by SIRT2 (I–IV). Wild-type spots were expressed as 100% (open bars), and the SD is given. Note that spots constituted by SIRT2 are virtually absent from PLP_null_ myelin (closed bars). D, Comparison of wild-type and PLP_null_ myelin by 2D-16-BAC/SDS-PAGE. Note that spots constituted by PLP and SIRT2 (left, arrows) are lacking from PLP_null_ myelin (right). E, Relative intensities of spots constituted by SEPT2, SEPT4, and SEPT8 in wild-type myelin (open bars) or PLP_null_ myelin (closed bars).
Figure 4.
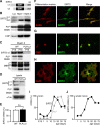
SIRT2 expression profile in myelinating glia. A, Two SIRT2 isoforms emerge by alternative splicing. Variant (v) 1 and v2 differ by a 37 amino acids domain at the N terminus, and both contain the enzymatically active SIRT-domain. B, 1D-PAGE/Western blot analysis of brain lysate (Lys) and myelin-enriched fractions (Myel 1 and Myel 2). The enrichment of SIRT2 with myelin purification was equivalent to that of the classical myelin proteins PLP/DM20 and CNP. Note that the two bands detected in brain lysate most likely represent the two splice isoforms, and that only v2 was enriched in myelin. C, 1D-PAGE/Western blot analysis of wild type (WT) and PLP_null_ myelin 2. SIRT2 and PLP were undetectable in PLP_null_ myelin 2, whereas MBP was unaltered. SIRT2 and PLP were equally abundant in wild-type myelin of young animals (P15) and adults (P75), whereas the abundance of certain MBP splice isoforms was developmentally regulated. D, 1D-PAGE/Western blot analysis of SIRT2 in wild-type and PLP_null_ brain lysates. Variant 1 was equally abundant in wild-type and PLP_null_, whereas variant 2 was virtually undetectable in PLP_null_ brains. E, Quantitative RT-PCR analysis of SIRT2 mRNA expression in adult (P75) wild-type (open bar) and PLP_null_ brains (closed bar). SIRT2 mRNA expression was unaltered. F–H, Immunodetection of SIRT2 (green) and differentiation markers (red) in cultured primary oligodendrocytes. A2B2 was used for oligodendrocyte precursors (F), CNP for differentiated oligodendrocytes (G), and GalC for more mature oligodendrocytes (H). I–J, Quantitative RT-PCR analysis of SIRT2 mRNA expression during brain development (I) or during sciatic nerve development (J). Maximum mRNA levels were arbitrarily set at 100%.
Figure 5.
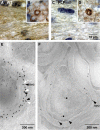
SIRT2 is a component of wild-type CNS myelin. A–D, Immunodetection of SIRT2 (brown; nuclei were counterstained in blue). Shown are representative images of the CNS white matter (coronal sections of the corpus callosum in A and C) or PNS (cross sections of the dorsal roots in B and D) from wild-type (A, B) and PLP_null_ (C, D) mice. Note that SIRT2 labeling marks all myelinated fibers in wild-type (A) but is essentially absent from mutant CNS myelin (C). Also note that SIRT2 expression in myelinating Schwann cells and enrichment in paranodes of PNS myelin is not dependent on PLP (B, D). E, Immunodetection of SIRT2 (10 nm gold particles; black arrowheads) together with PLP (15 nm gold particles; white arrowheads) on sections of cultured primary oligodendrocytes. Note that most SIRT2 labeling was in close proximity to PLP labeling and attached to the same membrane of a myelin-like multimembrane stack, possibly of endosomal origin. F, Immunodetection of SIRT2 visualized with gold particles in spinal cord cross sections. SIRT2 labeling was mostly confined to compact and adaxonal myelin (arrowheads). Axons and the extracellular space between myelin and axon were occasionally labeled (stars).
Figure 6.
Acetylated proteins in the myelin-enriched fraction. A, Western blot analysis of PLP_null_ myelin 2 with antibodies specific for acetylated lysine after 2D-IEF/SDS-PAGE. The major acetylated protein is boxed. B, The identity of the major acetylated protein (from A) as α-tubulin was suggested by spot pattern comparison with the myelin proteome reference map. The membrane was stripped and reprobed with antibodies specific for α-tubulin. Myelin was also separated in parallel for visualization by colloidal Coomassie stain (bottom). The spots at the corresponding positions were identified by MALDI-TOF-MS as α-tubulin. Note that several acetylated proteins were of too low abundance to constitute spots on Coomassie gels and could not be identified. C, Western blot analysis of PLP_null_ myelin 2 with antibodies specific for acetylated lysine after 2D-16-BAC/SDS-PAGE. Major acetylated proteins are boxed. D, E, The identity of the major acetylated proteins (from C) as α-tubulin and MOG was suggested by spot pattern comparison with the myelin proteome reference map. The membrane was stripped and reprobed with antibodies specific for α-tubulin (D) or MOG (E). Myelin was also separated in parallel for colloidal Coomassie stain, and the spots at the corresponding positions were identified by MALDI-TOF-MS as α-tubulin (D) and MOG (E). Note that membrane-spanning proteins (such as MOG) were only detected without previous isoelectric focusing (IEF). acLys, Acetylated lysine; Coom., colloidal Coomassie staining.
Similar articles
- The QKI-PLP pathway controls SIRT2 abundance in CNS myelin.
Zhu H, Zhao L, Wang E, Dimova N, Liu G, Feng Y, Cambi F. Zhu H, et al. Glia. 2012 Jan;60(1):69-82. doi: 10.1002/glia.21248. Epub 2011 Sep 21. Glia. 2012. PMID: 21948283 Free PMC article. - Myelin proteolipid proteins promote the interaction of oligodendrocytes and axons.
Yool DA, Klugmann M, McLaughlin M, Vouyiouklis DA, Dimou L, Barrie JA, McCulloch MC, Nave KA, Griffiths IR. Yool DA, et al. J Neurosci Res. 2001 Jan 15;63(2):151-64. doi: 10.1002/1097-4547(20010115)63:2<151::AID-JNR1007>3.0.CO;2-Y. J Neurosci Res. 2001. PMID: 11169625 - Oligodendrocytes expressing exclusively the DM20 isoform of the proteolipid protein gene: myelination and development.
Spörkel O, Uschkureit T, Büssow H, Stoffel W. Spörkel O, et al. Glia. 2002 Jan;37(1):19-30. doi: 10.1002/glia.10014. Glia. 2002. PMID: 11746780 - Phylogeny of proteolipid proteins: divergence, constraints, and the evolution of novel functions in myelination and neuroprotection.
Möbius W, Patzig J, Nave KA, Werner HB. Möbius W, et al. Neuron Glia Biol. 2008 May;4(2):111-27. doi: 10.1017/S1740925X0900009X. Epub 2009 Jun 5. Neuron Glia Biol. 2008. PMID: 19497142 Review. - Current concepts of PLP and its role in the nervous system.
Griffiths I, Klugmann M, Anderson T, Thomson C, Vouyiouklis D, Nave KA. Griffiths I, et al. Microsc Res Tech. 1998 Jun 1;41(5):344-58. doi: 10.1002/(SICI)1097-0029(19980601)41:5<344::AID-JEMT2>3.0.CO;2-Q. Microsc Res Tech. 1998. PMID: 9672418 Review.
Cited by
- CMTM6 expressed on the adaxonal Schwann cell surface restricts axonal diameters in peripheral nerves.
Eichel MA, Gargareta VI, D'Este E, Fledrich R, Kungl T, Buscham TJ, Lüders KA, Miracle C, Jung RB, Distler U, Kusch K, Möbius W, Hülsmann S, Tenzer S, Nave KA, Werner HB. Eichel MA, et al. Nat Commun. 2020 Sep 9;11(1):4514. doi: 10.1038/s41467-020-18172-7. Nat Commun. 2020. PMID: 32908139 Free PMC article. - SIRT2 Plays Significant Roles in Lipopolysaccharides-Induced Neuroinflammation and Brain Injury in Mice.
Wang B, Zhang Y, Cao W, Wei X, Chen J, Ying W. Wang B, et al. Neurochem Res. 2016 Sep;41(9):2490-500. doi: 10.1007/s11064-016-1981-2. Epub 2016 Jun 27. Neurochem Res. 2016. PMID: 27350577 - Aging-related rotenone-induced neurochemical and behavioral deficits: role of SIRT2 and redox imbalance, and neuroprotection by AK-7.
Wang X, Guan Q, Wang M, Yang L, Bai J, Yan Z, Zhang Y, Liu Z. Wang X, et al. Drug Des Devel Ther. 2015 May 7;9:2553-63. doi: 10.2147/DDDT.S81539. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26089639 Free PMC article. - Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways, and defense mechanisms.
Reumann S, Babujee L, Ma C, Wienkoop S, Siemsen T, Antonicelli GE, Rasche N, Lüder F, Weckwerth W, Jahn O. Reumann S, et al. Plant Cell. 2007 Oct;19(10):3170-93. doi: 10.1105/tpc.107.050989. Epub 2007 Oct 19. Plant Cell. 2007. PMID: 17951448 Free PMC article. - Sirt2 promotes white matter oligodendrogenesis during development and in models of neonatal hypoxia.
Jablonska B, Adams KL, Kratimenos P, Li Z, Strickland E, Haydar TF, Kusch K, Nave KA, Gallo V. Jablonska B, et al. Nat Commun. 2022 Aug 15;13(1):4771. doi: 10.1038/s41467-022-32462-2. Nat Commun. 2022. PMID: 35970992 Free PMC article.
References
- Almqvist P, Carlsson SR, Hardy JA, Winblad B. Regional and subcellular distribution of Thy-1 in human brain assayed by a solid-phase radioimmunoassay. J Neurochem. 1986;46:681–685. - PubMed
- Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer's disease. J Neurochem. 2006;96:305–313. - PubMed
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. - PubMed
- Barbarese E, Koppel DE, Deutscher MP, Smith CL, Ainger K, Morgan F, Carson JH. Protein translation components are colocalized in granules in oligodendrocytes. J Cell Sci. 1995;108:2781–2790. - PubMed
- Benjamins JA, Morell P. Proteins of myelin and their metabolism. Neurochem Res. 1978;3:137–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials