Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing - PubMed (original) (raw)
Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing
Michael R Drew et al. J Neurosci. 2007.
Abstract
The striatum receives prominent dopaminergic innervation that is integral to appetitive learning, performance, and motivation. Signaling through the dopamine D2 receptor is critical for all of these processes. For instance, drugs with high affinity for the D2 receptor potently alter timing of operant responses and modulate motivation. Recently, in an attempt to model a genetic abnormality encountered in schizophrenia, mice were generated that reversibly overexpress D2 receptors specifically in the striatum (Kellendonk et al., 2006). These mice have impairments in working memory and behavioral flexibility, components of the cognitive symptoms of schizophrenia, that are not rescued when D2 overexpression is reversed in the adult. Here we report that overexpression of striatal D2 receptors also profoundly affects operant performance, a potential index of negative symptoms. Mice overexpressing D2 exhibited impairments in the ability to time food rewards in an operant interval timing task and reduced motivation to lever press for food reward in both the operant timing task and a progressive ratio schedule of reinforcement. The motivational deficit, but not the timing deficit, was rescued in adult mice by reversing D2 overexpression with doxycycline. These results suggest that early D2 overexpression alters the organization of interval timing circuits and confirms that striatal D2 signaling in the adult regulates motivational process. Moreover, overexpression of D2 under pathological conditions such as schizophrenia and Parkinson's disease could give rise to motivational and timing deficits.
Figures
Figure 1.
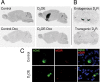
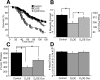
A, In situ hybridization against the transgenic D2 mRNA. Expression was detected in the striatum and olfactory tubercle of D2OE mice and was completely absent in D2OE-Dox and control mice. B, In situ hybridization of two adjacent 16 μm sections hybridized with an antisense oligonucleotide against the mouse D2 receptor coding sequence (top) or an antisense oligonucleotide directed against the transgenic D2 mRNA (bottom). Whereas endogenous D2 receptors are expressed in dopaminergic midbrain neurons, transgenic D2 receptors are absent. C, Double in situ hybridization using cRNA probes against acetylcholinesterase (AChE; left) and the transgenic D2 receptor (trD2R; middle). The right panel shows the overlay (with DAPI nuclear stain).
Figure 2.
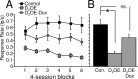
Mean response rate on FI trials as a function of session (A) and averaged across all sessions (B). Response rate was reduced in D2OE mice, and this reduction was partially ameliorated in D2OE-Dox mice. *p < 0.05. ns, Not significant.
Figure 3.
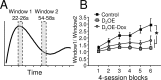
The development of operant response timing as a function of training session. A, To assess timing of operant responding, we compared the response rate during seconds 22–26 (Window 1) with that of seconds 54–58 (Window 2). B, The window 1/window 2 ratio increased as a function of sessions, but the increase was greater in control mice than in D2OE and D2OE-Dox mice. *p < 0.05.
Figure 4.
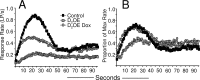
Timing of operant responses during the final four sessions of the peak interval procedure. A, Mean response rate during peak trials as a function of time in the trial. In B, response rates are expressed as a proportion of the per-trial maximum response rate. Both D2OE and D2OE-Dox mice have broader distributions of responding, consistent with reduced timing precision. Response rate was reduced in D2OE mice, and this deficit was partially rescued in D2OE-Dox mice.
Figure 5.
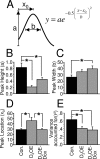
Mathematical modeling of peak interval performance. Peak trial data were fit to a Gaussian probability density function, illustrated in A. B–E show the mean best-fit parameter values from the Gaussian fits. B, Peak height was reduced in D2OE mice but rescued in D2OE-Dox mice. C, Peak width was significantly increased in D2OE-Dox mice; the D2OE group did not differ significantly from either of the other groups. D, Peak location was significantly increased (right-shifted) in D2OE mice, and this increase was rescued in D2OE-Dox mice. E, The Gaussian function provided a better fit for the control data than for data from either D2OE or D2OE-Dox mice. *p < 0.05.
Figure 6.
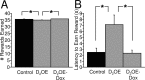
On FI trials, mean number of rewards earned per session (A) and mean latency to earn the reward after the FI had elapsed (B). D2OE mice earned slightly but significantly fewer rewards and took longer to earn rewards than the other groups. Both of these deficits were rescued in the D2OE-Dox mice. *p < 0.05.
Figure 7.
Performance in the progressive ratio task. A, Kapan–Meier survival function plotting the percentage of subjects continuing to respond on the progressive ratio schedule as a function of session time. D2OE mice ceased responding significantly sooner than the other groups. B, Mean number of rewards earned per session and break point (last ratio completed). D2OE mice earned fewer rewards and had a lower break point than the other groups. C, Mean number of lever presses per session. D2OE and D2OE-Dox mice made fewer responses than controls. The difference between D2OE and D2OE-Dox approached significance (p = 0.08). D, Mean number of head entries to the food compartment while food rewards were available (i.e., the dipper was up). The groups did not differ on this measure, indicating that all groups approached rewards when they were available. *p < 0.05.
Figure 8.
Preference for sucrose solution in the two-bottle choice test. Amount of sucrose solution consumed is expressed as a proportion of total liquid consumed (sucrose plus water). Both control and D2OE mice exhibited a strong preference for the sucrose solution that was not affected by food deprivation.
Similar articles
- Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning.
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Kellendonk C, et al. Neuron. 2006 Feb 16;49(4):603-15. doi: 10.1016/j.neuron.2006.01.023. Neuron. 2006. PMID: 16476668 - Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits.
Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, Kandel ER, Balsam PD. Ward RD, et al. Behav Neurosci. 2009 Aug;123(4):720-30. doi: 10.1037/a0016503. Behav Neurosci. 2009. PMID: 19634929 Free PMC article. - The impact of motivation on cognitive performance in an animal model of the negative and cognitive symptoms of schizophrenia.
Ward RD, Winiger V, Higa KK, Kahn JB, Kandel ER, Balsam PD, Simpson EH. Ward RD, et al. Behav Neurosci. 2015 Jun;129(3):292-9. doi: 10.1037/bne0000051. Epub 2015 Apr 27. Behav Neurosci. 2015. PMID: 25914923 Free PMC article. - Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine.
Salamone JD, Correa M. Salamone JD, et al. Behav Brain Res. 2002 Dec 2;137(1-2):3-25. doi: 10.1016/s0166-4328(02)00282-6. Behav Brain Res. 2002. PMID: 12445713 Review. - Schizophrenia in translation: dissecting motivation in schizophrenia and rodents.
Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Simpson EH, et al. Schizophr Bull. 2012 Nov;38(6):1111-7. doi: 10.1093/schbul/sbs114. Epub 2012 Sep 26. Schizophr Bull. 2012. PMID: 23015686 Free PMC article. Review.
Cited by
- Neuropsychopharmacology and neurogenetic aspects of executive functioning: should reward gene polymorphisms constitute a diagnostic tool to identify individuals at risk for impaired judgment?
Bowirrat A, Chen TJ, Oscar-Berman M, Madigan M, Chen AL, Bailey JA, Braverman ER, Kerner M, Giordano J, Morse S, Downs BW, Waite RL, Fornari F, Armaly Z, Blum K. Bowirrat A, et al. Mol Neurobiol. 2012 Apr;45(2):298-313. doi: 10.1007/s12035-012-8247-z. Epub 2012 Feb 28. Mol Neurobiol. 2012. PMID: 22371275 Free PMC article. Review. - Insights About Striatal Circuit Function and Schizophrenia From a Mouse Model of Dopamine D2 Receptor Upregulation.
Simpson EH, Kellendonk C. Simpson EH, et al. Biol Psychiatry. 2017 Jan 1;81(1):21-30. doi: 10.1016/j.biopsych.2016.07.004. Epub 2016 Jul 14. Biol Psychiatry. 2017. PMID: 27720388 Free PMC article. Review. - A Touchscreen Motivation Assessment Evaluated in Huntington's Disease Patients and R6/1 Model Mice.
Heath CJ, O'Callaghan C, Mason SL, Phillips BU, Saksida LM, Robbins TW, Barker RA, Bussey TJ, Sahakian BJ. Heath CJ, et al. Front Neurol. 2019 Aug 9;10:858. doi: 10.3389/fneur.2019.00858. eCollection 2019. Front Neurol. 2019. PMID: 31447770 Free PMC article. - Modeling motivational deficits in mouse models of schizophrenia: behavior analysis as a guide for neuroscience.
Ward RD, Simpson EH, Kandel ER, Balsam PD. Ward RD, et al. Behav Processes. 2011 May;87(1):149-56. doi: 10.1016/j.beproc.2011.02.004. Epub 2011 Feb 18. Behav Processes. 2011. PMID: 21338658 Free PMC article. Review. - Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning.
Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Bach ME, et al. Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):16027-32. doi: 10.1073/pnas.0807746105. Epub 2008 Oct 2. Proc Natl Acad Sci U S A. 2008. PMID: 18832466 Free PMC article.
References
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. - PubMed
- Artieda J, Pastor MA, Lacruz F, Obeso JA. Temporal discrimination is abnormal in Parkinson's disease. Brain. 1992;115:199–210. - PubMed
- Balsam PD, Drew MR, Yang C. Timing at the start of associative learning. Learn Motiv. 2002;33:141–155.
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases