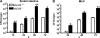MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection - PubMed (original) (raw)
MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection
Katharina Brandl et al. J Exp Med. 2007.
Abstract
Listeria monocytogenes is a food-borne bacterial pathogen that causes systemic infection by traversing the intestinal mucosa. Although MyD88-mediated signals are essential for defense against systemic L. monocytogenes infection, the role of Toll-like receptor and MyD88 signaling in intestinal immunity against this pathogen has not been defined. We show that clearance of L. monocytogenes from the lumen of the distal small intestine is impaired in MyD88(-/-) mice. The distal ileum of wild-type (wt) mice expresses high levels of RegIII gamma, which is a bactericidal lectin that is secreted into the bowel lumen, whereas RegIII gamma expression in MyD88(-/-) mice is nearly undetectable. In vivo depletion of RegIII gamma from the small intestine of wt mice diminishes killing of luminal L. monocytogenes, whereas reconstitution of MyD88-deficient mice with recombinant RegIII gamma enhances intestinal bacterial clearance. Experiments with bone marrow chimeric mice reveal that MyD88-mediated signals in nonhematopoietic cells induce RegIII gamma expression in the small intestine, thereby enhancing bacterial killing. Our findings support a model of MyD88-mediated epithelial conditioning that protects the intestinal mucosa against bacterial invasion by inducing RegIII gamma.
Figures
Figure 1.
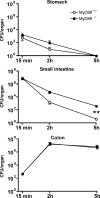
L. monocytogenes transit through the gastrointestinal tract after intragastric inoculation of MyD88+/+ and MyD88−/− mice. Quantitation of L. monocytogenes in stomach (wall + lumen), small intestine (wall + lumen), and colon (wall + lumen) in MyD88+/+ and MyD88−/− mice after oral infection with 109 L. monocytogenes, 15, 120, and 300 min p.i. Values are representative of two individual experiments with n = 3 in each group. **, P < 0.01. Error bars represent the SD.
Figure 2.
MyD88-deficient mice are more susceptible to intestinal L. monocytogenes infection. MyD88+/+ and MyD88−/− mice were infected with 109 L. monocytogenes by gastric gavage and the number of bacteria in the small intestinal wall (A) and MLNs (B) was determined 12 (for small intestine), 24, 48, and 72 h after infection; n = 5–8 mice/group. *, P ≤ 0.05; **, P < 0.01; ND = not detectable. Error bars represent the SD.
Figure 3.
Diminished clearance of L. monocytogenes in the distal small intestine of MyD88-deficient mice. (A) MyD88+/+ and MyD88−/− mice were infected with 1010 L. monocytogenes by gavage, and CFUs in the proximal (pro) and distal part of the small intestinal wall were determined 24 h p.i.; n = 8 mice/group. (B) Segments from proximal (pro) or distal small intestine were cultured in media containing 2,000 L. monocytogenes. After 4 h, bacterial burden in the supernatant was determined. Values are representative of two individual experiments. (C) Schematic representation of the in vivo luminal killing assay. (D) Bioluminescence imaging of luminescent L. monocytogenes in ileal loops of MyD88+/+ and MyD88−/− mice. Luminescent L. monocytogenes (2.5 × 105) was injected into ileal loops of MyD88+/+ and MyD88−/− mice and imaged 30 min (top) and 2 h (bottom) after injection with an IVIS Imaging System. One representative mouse per group is shown. n = 5. (E) 1,000 L. monocytogenes were injected into ileal loops of wt, MyD88−/−, TNF−/−/IFN-γ−/−, and Rip2−/− mice. 2 h after injection, the isolated section of the intestine was harvested and CFUs in the luminal fluid were detected. n = 3 for MyD88−/−; n = 5–9 for all other groups. Bacterial numbers are expressed as the percentage of recovered L. monocytogenes. *, P ≤ 0.05, ***, P < 0.001. Error bars represent the SD.
Figure 4.
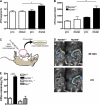
RegIIIγ expression is dependent on MyD88-mediated signals. mRNA was extracted from the terminal ileum of MyD88+/+ and MyD88−/− mice. Defcr-rs 1, Defcr 4, Defcr 5, Defcr-rs 10 (A), and RegIIIγ (B) expression were examined by quantitative real-time PCR. Expression levels were normalized to GAPDH. Values are representative of two experiments with two mice per group. (C) Protein extracts from the proximal (pro) and distal (dis) small intestine of MyD88+/+ and MyD88−/− mice were analyzed by Western blotting with RegIIIγ-specific antiserum. Tubulin was used as a loading control. In D, mice were infected with 109 L. monocytogenes by gavage, and mRNA was prepared before and 24 h after infection from the terminal ileum of wt (left) and MyD88-deficient (right) mice. Expression was examined by quantitative real-time PCR and levels were normalized to GAPDH. Values are representative of two experiments with two mice per group. Error bars represent the SD.
Figure 5.
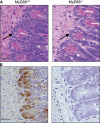
Histology of MyD88+/+ and MyD88-deficient mice. (A) Two representative small intestinal sections from the distal part of MyD88+/+ and MyD88−/− mice (hematoxylin and eosin–stained) are shown. Arrows indicate Paneth cells. (B) Immunohistochemical detection of RegIIIγ in paraffin-embedded distal small intestinal sections in MyD88+/+ and MyD88-deficient mice. Bars, 50 μm.
Figure 6.
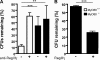
RegIIIγ is responsible for diminished luminal killing in MyD88-deficient mice. (A) Antiserum against RegIIIγ or the preimmune serum was injected into ileal loops of MyD88+/+ and MyD88−/− mice before injection of 1,000 L. monocytogenes. After 2 h, bacterial growth in the luminal fluid of the isolated section of the small intestine was determined; n = 6 mice/group. (B) After reconstitution of MyD88-deficient mice with recombinant RegIIIγ or control protein (β2microglobulin), killing of 1,000 L. monocytogenes in ileal loops of MyD88-deficient mice was assessed; n = 5 each group. (A and B) Bacterial numbers are expressed as the percentage of recovered L. monocytogenes. **, P < 0.01; ***, P < 0.0001. Error bars represent the SD.
Figure 7.
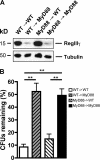
Nonhematopoietic MyD88-mediated signaling is involved in RegIIIγ expression and RegIIIγ-mediated luminal killing of L. monocytogenes. (A) Protein extracts from distal small intestine of chimeric mice were analyzed by Western blotting with RegIIIγ-specific antiserum. Tubulin was used as a loading control. Shown are representative extracts from each genotype. n = 5 for wt→MyD88 and for MyD88→wt; n = 3 for wt →wt and for MyD88→MyD88. (B) 1,000 L. monocytogenes were injected into ileal loops of different chimeric mice. After 2 h, bacterial growth in the luminal fluid of the isolated section of the small intestine was determined; n = 5 for wt→MyD88 and for MyD88→wt; n = 3 for wt →wt and for MyD88→MyD88. Bacterial numbers are expressed as the percentage of recovered L. monocytogenes. **, P < 0.01. Error bars represent the SD.
Similar articles
- Dendritic cells coordinate innate immunity via MyD88 signaling to control Listeria monocytogenes infection.
Arnold-Schrauf C, Dudek M, Dielmann A, Pace L, Swallow M, Kruse F, Kühl AA, Holzmann B, Berod L, Sparwasser T. Arnold-Schrauf C, et al. Cell Rep. 2014 Feb 27;6(4):698-708. doi: 10.1016/j.celrep.2014.01.023. Epub 2014 Feb 13. Cell Rep. 2014. PMID: 24529704 - MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection.
Jia T, Leiner I, Dorothee G, Brandl K, Pamer EG. Jia T, et al. J Immunol. 2009 Jul 15;183(2):1271-8. doi: 10.4049/jimmunol.0900460. Epub 2009 Jun 24. J Immunol. 2009. PMID: 19553532 Free PMC article. - Postoperative ileus involves interleukin-1 receptor signaling in enteric glia.
Stoffels B, Hupa KJ, Snoek SA, van Bree S, Stein K, Schwandt T, Vilz TO, Lysson M, Veer CV, Kummer MP, Hornung V, Kalff JC, de Jonge WJ, Wehner S. Stoffels B, et al. Gastroenterology. 2014 Jan;146(1):176-87.e1. doi: 10.1053/j.gastro.2013.09.030. Epub 2013 Sep 22. Gastroenterology. 2014. PMID: 24067878 - Tracing innate immune defences along the path of Listeria monocytogenes infection.
Regan T, MacSharry J, Brint E. Regan T, et al. Immunol Cell Biol. 2014 Aug;92(7):563-9. doi: 10.1038/icb.2014.27. Epub 2014 Apr 15. Immunol Cell Biol. 2014. PMID: 24732075 Review. - Understanding how Listeria monocytogenes targets and crosses host barriers.
Lecuit M. Lecuit M. Clin Microbiol Infect. 2005 Jun;11(6):430-6. doi: 10.1111/j.1469-0691.2005.01146.x. Clin Microbiol Infect. 2005. PMID: 15882192 Review.
Cited by
- No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens.
Sassone-Corsi M, Raffatellu M. Sassone-Corsi M, et al. J Immunol. 2015 May 1;194(9):4081-7. doi: 10.4049/jimmunol.1403169. J Immunol. 2015. PMID: 25888704 Free PMC article. Review. - The Potential Role of the Intestinal Micromilieu and Individual Microbes in the Immunobiology of Chimeric Antigen Receptor T-Cell Therapy.
Schubert ML, Rohrbach R, Schmitt M, Stein-Thoeringer CK. Schubert ML, et al. Front Immunol. 2021 May 31;12:670286. doi: 10.3389/fimmu.2021.670286. eCollection 2021. Front Immunol. 2021. PMID: 34135898 Free PMC article. Review. - Antibiotics, microbiota, and immune defense.
Ubeda C, Pamer EG. Ubeda C, et al. Trends Immunol. 2012 Sep;33(9):459-66. doi: 10.1016/j.it.2012.05.003. Epub 2012 Jun 5. Trends Immunol. 2012. PMID: 22677185 Free PMC article. Review. - Mechanisms of intestinal dysbiosis: new insights into tuft cell functions.
Coutry N, Gasmi I, Herbert F, Jay P. Coutry N, et al. Gut Microbes. 2024 Jan-Dec;16(1):2379624. doi: 10.1080/19490976.2024.2379624. Epub 2024 Jul 23. Gut Microbes. 2024. PMID: 39042424 Free PMC article. Review. - Prior exposure to microcystin alters host gut resistome and is associated with dysregulated immune homeostasis in translatable mouse models.
Saha P, Bose D, Stebliankin V, Cickovski T, Seth RK, Porter DE, Brooks BW, Mathee K, Narasimhan G, Colwell R, Scott GI, Chatterjee S. Saha P, et al. Sci Rep. 2022 Jul 7;12(1):11516. doi: 10.1038/s41598-022-15708-3. Sci Rep. 2022. PMID: 35799048 Free PMC article.
References
- Gellin, B.G., and C.V. Broome. 1989. Listeriosis. JAMA. 261:1313–1320. - PubMed
- Southwick, F.S., and D.L. Purich. 1996. Intracellular pathogenesis of listeriosis. N. Engl. J. Med. 334:770–776. - PubMed
- Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 292:1722–1725. - PubMed
- Mengaud, J., H. Ohayon, P. Gounon, R.M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 84:923–932. - PubMed
- Cossart, P., and P.J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 304:242–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI39031/AI/NIAID NIH HHS/United States
- R01 AI039031/AI/NIAID NIH HHS/United States
- R37 AI039031/AI/NIAID NIH HHS/United States
- AI42135/AI/NIAID NIH HHS/United States
- R01 AI042135/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases