ASB4 is a hydroxylation substrate of FIH and promotes vascular differentiation via an oxygen-dependent mechanism - PubMed (original) (raw)
ASB4 is a hydroxylation substrate of FIH and promotes vascular differentiation via an oxygen-dependent mechanism
James E Ferguson 3rd et al. Mol Cell Biol. 2007 Sep.
Abstract
The molecular mechanisms of endothelial differentiation into a functional vascular network are incompletely understood. To identify novel factors in endothelial development, we used a microarray screen with differentiating embryonic stem (ES) cells that identified the gene for ankyrin repeat and SOCS box protein 4 (ASB4) as the most highly differentially expressed gene in the vascular lineage during early differentiation. Like other SOCS box-containing proteins, ASB4 is the substrate recognition molecule of an elongin B/elongin C/cullin/Roc ubiquitin ligase complex that mediates the ubiquitination and degradation of substrate protein(s). High levels of ASB4 expression in the embryonic vasculature coincide with drastic increases in oxygen tension as placental blood flow is initiated. However, as vessels mature and oxygen levels stabilize, ASB4 expression is quickly downregulated, suggesting that ASB4 may function to modulate an endothelium-specific response to increasing oxygen tension. Consistent with the hypothesis that ASB4 function is regulated by oxygen concentration, ASB4 interacts with the factor inhibiting HIF1alpha (FIH) and is a substrate for FIH-mediated hydroxylation via an oxygen-dependent mechanism. Additionally, overexpression of ASB4 in ES cells promotes differentiation into the vascular lineage in an oxygen-dependent manner. We postulate that hydroxylation of ASB4 in normoxia promotes binding to and degradation of substrate protein(s) to modulate vascular differentiation.
Figures
FIG. 1.
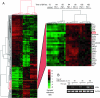
ASB4 is highly differentially expressed in the vascular lineage during ES cell differentiation (Diff). (A) Supervised hierarchical cluster of genes with a ≥1.5-fold absolute mean difference in 84-h Flk1+ cells from embryoid bodies. The Flk1 cluster containing ASB4 is shown at the right. This experiment was described in detail by Wang et al. (46), and the complete data set is available online through the Gene Expression Omnibus (series record GSE3757,
http://www.ncbi.nlm.nih.gov/geo
) and through the University of North Carolina Microarray Database (
). Experiments were performed in quadruplicate. (B) RT-PCR analysis of ASB4 expression levels in Flk1+ cells from differentiated embryoid bodies. GAPDH was used as a loading control. US, unsorted.
FIG. 2.
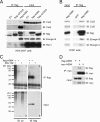
ASB4 associates with a ubiquitin ligase complex. (A) HEK-293T cells were transfected with Flag-ASB4, Flag-ASB4ΔSOCS (flag-ΔSOCS), or the EV pCMV. Lysates were immunoprecipitated (IP) with anti-Flag-agarose beads and subjected to immunoblotting (IB) with anti-Flag, -Cul2, -Cul5, -elongin B, and -Roc1 antibodies. Asterisks denote nonspecific bands. (B) COS7 cells were infected with Flag-ASB4 adenovirus or GFP-alone adenovirus (GFP). Lysates were immunoprecipitated and immunoblotted as described for panel A. (C) HEK-293T cells were transfected with Flag-ASB4, cultured for 20 h, and then treated with the proteasome inhibitor MG-132 at 40 μM for 4 h before protein harvesting. Lysates were immunoprecipitated as described for panel A and immunoblotted with antiubiquitin (ub) and anti-Flag antibodies. (D) HEK-293T cells were cotransfected with Flag-ASB4 and myc-ASB4, immunoprecipitated with anti-myc agarose beads, and immunoblotted.
FIG. 3.
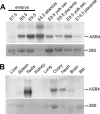
ASB4 mRNA expression in embryonic and adult tissues. (A) Northern blot analysis of ASB4 expression levels in whole embryos and dissected extraembryonic tissues. (B) Northern blot analysis of ASB4 expression levels in adult tissues. BM, bone marrow. Ethidium bromide staining of 28S rRNA was used as a loading control.
FIG. 4.
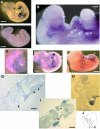
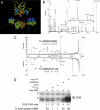
Localization of ASB4 mRNA during mouse embryogenesis. Whole-mount in situ hybridization with DIG-labeled RNA probes for ASB4 was performed on E9.5 to E11.5 mouse embryos. (A) E9.5 embryo. Arrow, allantois; arrowhead, forelimb; bar, 500 μm. (B) E9.5 embryo at high magnification. Arrow, rostral capillary plexus; arrowheads, branchial arch capillary plexi; bar, 50 μm. (C) E9.5 embryo probed with sense probe as a negative control. Bar, 500 μm. (D) E9.5 yolk sac. Arrows, yolk sac vessels; bar, 750 μm. (E) E10.5 embryo. Arrow, umbilical vessels; bar, 800 μm. (F) E11.5 embryo. Arrow, caudal intersomitic vessels; bar, 1 mm. (G) Transverse section of E9.5 embryo heart. Arrow, endocardium; arrowheads, dorsal aorta and intersomitic vessel; bar, 80 μm. (H) Sagittal section of E9.5 embryo heart. v, ventricle; arrow, pro-epicardium/septum transversum. (I) Transverse section of E10.5 embryo liver. HL, hind limb; FL, forelimb; arrow, liver; arrowhead, umbilical vessels; bar, 150 μm. (J) Schematic of section location in panels G and I. Antisense probes were used for all images except that in panel C (sense probe). Purple staining denotes a positive signal. In some cases (G, H, and I), stained whole embryos were paraffin embedded and sectioned for microscopic examination.
FIG. 5.
ASB4 binds to FIH through a conserved motif in AR6. (A) Yeast wheel assay. Yeast cells transformed with different combinations of bait (ASB4ΔSOCS) and prey (FIH) constructs were spread onto a high-stringency selective plate lacking tryptophan, leucine, histidine, and adenine. p53 and T were used as positive controls. V1, empty bait vector (pGBKT7). V2, empty prey vector (pGADT7). (B) HEK-293T cells were transfected with either Flag-ASB4 or Flag-ASB1. Lysates were immunoprecipitated (IP) with Flag antibody-conjugated agarose beads and immunoblotted (IB) with anti-Flag and anti-FIH antibodies. (C) Sequence alignment of mouse ASB4 and other known FIH hydroxylation substrates. The consensus sequence shows high conservation of E244, V245, N246, A247, and flanking leucine residues. Residue numbers are indicated above the ASB4 sequence. (D) Schematic of ASB4 with the N-terminal variable region (NTV), nine tandem ARs (poorly conserved repeats are indicated by parentheses), and a C-terminal SOCS box. Asparagine 246 is contained in the loop of AR6. Schematics of N-terminally Flag-tagged constructs are denoted below. (E) HEK-293T cells were transfected with Flag-ASB4, Flag-ASB1, and the Flag-ASB4 deletion mutants described in panel D. Protein lysates were immunoprecipitated with anti-Flag-agarose and immunoblotted with anti-FIH antibody. (F) HEK-293T cells were transfected with various Flag-ASB4 point mutants and myc-FIH. Lysates were immunoprecipitated with Flag antibody-conjugated agarose beads and immunoblotted with anti-Flag and anti-myc antibodies. WCL, whole-cell lysate.
FIG. 6.
ASB4 is hydroxylated on asparagine 246 by an oxygen-dependent mechanism. (A) Structural model of ASB4 AR6 and -7 (yellow ribbon backbone) positioned above the FIH (gray stick backbone)/HIF peptide (purple ribbon backbone) crystal structure (PDB accession no. 1H2K). Space-filled EVNA residues are colored orange. Space-filled FIH residues contributing to HIF binding are colored yellow. Leucines and other possible hydrophobic interacting residues are colored blue. (B) COS7 cells were infected with Flag-ASB4 adenovirus for 24 h. Lysates were immunoprecipitated with Flag antibody-conjugated agarose beads. Beads were eluted with Flag-tagged peptide and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie staining. Flag-ASB4 bands were subjected to in-gel trypsin digestion and MALDI-TOF MS analysis. The 1,329-Da peak represents the unhydroxylated form of the asparagine-containing peptide, and the 1,345-Da peak (asterisk) represents the hydroxylated form of the peptide. (C) The 1,329- and 1,345-Da peptides were analyzed by MS/MS. A 16-Da shift was observed with the y4-y8 ions but not with the y2 ions, consistent with hydroxylation at the asparagine residue. (D) HEK-293T cells transiently transfected with Flag-ASB4 were cotransfected with myc-FIH or siRNA targeting FIH or GAPDH (negative control) or cultured under hypoxic conditions (1%). Flag-ASB4 protein was isolated and analyzed as for panel B, and the peak area ratio of unhydroxylated (1,329 Da) to hydroxylated (1,345 Da) peptide was calculated to evaluate the percentage of hydroxylated ASB4. In parallel, whole-cell lysates were subjected to immunoblotting (IB) by FIH antibody. Asterisks indicate nonspecific bands.
FIG. 7.
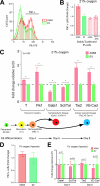
ASB4 overexpression in ES cells increases the percentage of Flk1+ cells and vascular commitment via an oxygen-dependent mechanism. ES cells were electroporated with constructs encoding cytomegalovirus promoter-driven 3×Flag-ASB4, unhydroxylatable ASB4 mutants (ΔAR6 and EVN→ATA), or the vector alone (EV), and stably expressing clones were selected for by culture in G418 for 14 days. Positive clones were isolated, expanded, and used for differentiation experiments as previously described. (A and B) FACS analysis of 96-h differentiated embryoid bodies (EBs) shows a drastic increase in Flk1+ cells in ASB4-expressing clones but not in clones expressing unhydroxylatable mutants. Results in panel A are representative of three independent experiments. Results in panel B represent averages of three independent clones in three independent differentiation experiments. Flk1+ cells in ASB4-expressing clones were normalized to EV clones in each experiment. (C) Real-time RT-PCR analysis of differentiated embryoid bodies was used to determine the lineage commitment of ASB4-expressing ES cells. Compared with EV cells, 96-h differentiated ASB4-expressing ES cells show increased expression of a marker of mesoderm commitment (brachyury [T]) and of a marker of early vascular lineage commitment (Flk1). At day 6, ASB4-expressing ES cells show decreased expression of hematopoietic lineage markers (Gata1, Scl/Tal), and at day 8, they show increased expression of vascular markers (Tie2, VE-cadherin [VE-Cad]). Results represent averages of three independent biologic replicates. (D and E) Embryoid bodies stably expressing either ASB4 or EV were differentiated under hypoxic conditions (1% oxygen) and analyzed via FACS and real-time PCR as described for panels B and C.
Similar articles
- The ubiquitin ligase ASB4 promotes trophoblast differentiation through the degradation of ID2.
Townley-Tilson WH, Wu Y, Ferguson JE 3rd, Patterson C. Townley-Tilson WH, et al. PLoS One. 2014 Feb 21;9(2):e89451. doi: 10.1371/journal.pone.0089451. eCollection 2014. PLoS One. 2014. PMID: 24586788 Free PMC article. - MYPT1, the targeting subunit of smooth-muscle myosin phosphatase, is a substrate for the asparaginyl hydroxylase factor inhibiting hypoxia-inducible factor (FIH).
Webb JD, Murányi A, Pugh CW, Ratcliffe PJ, Coleman ML. Webb JD, et al. Biochem J. 2009 May 13;420(2):327-33. doi: 10.1042/BJ20081905. Biochem J. 2009. PMID: 19245366 - Regulation of ankyrin repeat and suppressor of cytokine signalling box protein 4 expression in the immortalized murine endothelial cell lines MS1 and SVR: a role for tumour necrosis factor alpha and oxygen.
Bode M, Wu Y, Pi X, Lockyer P, Dechyapirom W, Portbury AL, Patterson C. Bode M, et al. Cell Biochem Funct. 2011 Jun;29(4):334-41. doi: 10.1002/cbf.1755. Cell Biochem Funct. 2011. PMID: 21506136 Free PMC article. - The SOCS box-adapting proteins for ubiquitination and proteasomal degradation.
Linossi EM, Nicholson SE. Linossi EM, et al. IUBMB Life. 2012 Apr;64(4):316-23. doi: 10.1002/iub.1011. Epub 2012 Feb 23. IUBMB Life. 2012. PMID: 22362562 Review. - The Roles of Obesity and ASB4 in Preeclampsia Pathogenesis.
Wang Y, Ssengonzi R, Townley-Tilson WHD, Kayashima Y, Maeda-Smithies N, Li F. Wang Y, et al. Int J Mol Sci. 2024 Aug 20;25(16):9017. doi: 10.3390/ijms25169017. Int J Mol Sci. 2024. PMID: 39201703 Free PMC article. Review.
Cited by
- Factor inhibiting HIF can catalyze two asparaginyl hydroxylations in VNVN motifs of ankyrin fold proteins.
Leissing TM, Hardy AP, Chan H, Wang Y, Tumber A, Chowdhury R, Feng T, Coleman ML, Cockman ME, Kramer HB, Berridge G, Fischer R, Kessler BM, Ratcliffe PJ, Lu X, Schofield CJ. Leissing TM, et al. J Biol Chem. 2022 Jun;298(6):102020. doi: 10.1016/j.jbc.2022.102020. Epub 2022 May 7. J Biol Chem. 2022. PMID: 35537551 Free PMC article. - The Lineage Differentiation and Dynamic Heterogeneity of Thymic Epithelial Cells During Thymus Organogenesis.
Gao H, Cao M, Deng K, Yang Y, Song J, Ni M, Xie C, Fan W, Ou C, Huang D, Lin L, Liu L, Li Y, Sun H, Cheng X, Wu J, Xia C, Deng X, Mou L, Chen P. Gao H, et al. Front Immunol. 2022 Feb 22;13:805451. doi: 10.3389/fimmu.2022.805451. eCollection 2022. Front Immunol. 2022. PMID: 35273595 Free PMC article. - Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease.
Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Willis MS, et al. Circ Res. 2010 Feb 19;106(3):463-78. doi: 10.1161/CIRCRESAHA.109.208801. Circ Res. 2010. PMID: 20167943 Free PMC article. Review. - The effect of human embryonic stem cells (hESCs) long-term normoxic and hypoxic cultures on the maintenance of pluripotency.
Zachar V, Prasad SM, Weli SC, Gabrielsen A, Petersen K, Petersen MB, Fink T. Zachar V, et al. In Vitro Cell Dev Biol Anim. 2010 Apr;46(3-4):276-83. doi: 10.1007/s11626-010-9305-3. Epub 2010 Feb 23. In Vitro Cell Dev Biol Anim. 2010. PMID: 20177991 - Quantitative mass spectrometry reveals dynamics of factor-inhibiting hypoxia-inducible factor-catalyzed hydroxylation.
Singleton RS, Trudgian DC, Fischer R, Kessler BM, Ratcliffe PJ, Cockman ME. Singleton RS, et al. J Biol Chem. 2011 Sep 30;286(39):33784-94. doi: 10.1074/jbc.M111.262808. Epub 2011 Jul 30. J Biol Chem. 2011. PMID: 21808058 Free PMC article.
References
- Cockman, M. E., D. E. Lancaster, I. P. Stolze, K. S. Hewitson, M. A. McDonough, M. L. Coleman, C. H. Coles, X. Yu, R. T. Hay, S. C. Ley, C. W. Pugh, N. J. Oldham, N. Masson, C. J. Schofield, and P. J. Ratcliffe. 2006. Posttranslational hydroxylation of ankyrin repeats in IκB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH). Proc. Natl. Acad. Sci. USA 103:14767-14772. - PMC - PubMed
- Cross, J. C. 2006. Placental function in development and disease. Reprod. Fertil. Dev. 18:71-76. - PubMed
- Debrincat, M. A., J. G. Zhang, T. A. Willson, J. Silke, L. M. Connolly, R. J. Simpson, W. S. Alexander, N. A. Nicola, B. T. Kile, and D. J. Hilton. 2007. Ankyrin repeat and suppressors of cytokine signaling box containing protein Asb-9 targets creatine kinase B for degradation. J. Biol. Chem. 282:4728-4737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL061656/HL/NHLBI NIH HHS/United States
- R01 HL072347/HL/NHLBI NIH HHS/United States
- HL 072347/HL/NHLBI NIH HHS/United States
- HL 61656/HL/NHLBI NIH HHS/United States
- HL 03658/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases