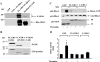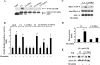GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability - PubMed (original) (raw)
GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability
Nebojsa Knezevic et al. Mol Cell Biol. 2007 Sep.
Abstract
We identified the GDI-1-regulated mechanism of RhoA activation from the Rho-GDI-1 complex and its role in mediating increased endothelial permeability. Thrombin stimulation failed to induce RhoA activation and actin stress fiber formation in human pulmonary arterial endothelial cells transduced with full-length GDI-1. Expression of a GDI-1 mutant form (C-GDI) containing the C terminus (aa 69 to 204) also prevented RhoA activation, whereas further deletions failed to alter RhoA activation. We observed that protein kinase Calpha-mediated phosphorylation of the C terminus of GDI-1 at Ser96 reduced the affinity of GDI-1 for RhoA and thereby enabled RhoA activation. Rendering GDI-1 phosphodefective with a Ser96 --> Ala substitution rescued the inhibitory activity of GDI-1 toward RhoA but did not alter the thrombin-induced activation of other Rho GTPases, i.e., Rac1 and Cdc42. Phosphodefective mutant GDI-1 also suppressed myosin light chain phosphorylation, actin stress fiber formation, and the increased endothelial permeability induced by thrombin. In contrast, expressing the phospho-mimicking mutant S96D-GDI-1 protein induced RhoA activity and increased endothelial permeability independently of thrombin stimulation. These results demonstrate the crucial role of the phosphorylation of the C terminus of GDI-1 at S96 in selectively activating RhoA. Inhibiting GDI-1 phosphorylation at S96 is a potential therapeutic target for modulating RhoA activity and thus preventing the increase in endothelial permeability associated with vascular inflammation.
Figures
FIG. 1.
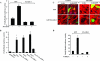
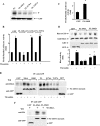
GDI-1 expression prevents SRE activation and actin stress fiber formation. (A) Effect of GDI-1 on SRE activity induced by thrombin. HPAE cells were cotransfected with an SRE-luciferase plasmid and GFP or GFP-tagged FL GDI-1. Cells were then stimulated with thrombin for 5 h prior to SRE activity measurement with a dual-reporter assay kit. SRE-luciferase activity is expressed as the ratio of firefly to Renilla luciferase activities. Data represent the mean ± the standard deviation from four experiments performed in triplicate. Asterisks indicate values different from those obtained with unstimulated vector-expressing cells or cells expressing FL GDI-1 with or without thrombin stimulation (P < 0.05); +, presence; −, absence. (B) Effect of GDI-1 on SRE activity induced by constitutively active heterotrimeric G proteins. HPAE cells were cotransfected with constitutively active Gαq, Gα12, or Gα13 mutant with or without FL GDI-1, and SRE reporter activity was determined. SRE-Luc activity is expressed as the ratio of firefly to Renilla luciferase activities. Data represent the mean ± the standard deviation from three experiments performed in triplicate. Asterisks indicate values different from those obtained with vector-expressing cells or cells coexpressing FL GDI-1 and constitutively active Gα subunits (P < 0.05). (C) Effect of GDI-1 on thrombin-induced actin stress fiber formation. HPAE cells transfected with GFP or GFP-GDI-1 were stimulated with 50 nM thrombin for 5 min, after which cells were fixed and stained with phalloidin to determine actin stress fiber formation. Results are representative of at least three experiments. (D) Effect of GDI-1 on SRE activation induced by constitutively active RhoA. HPAE cells were cotransfected with constitutively active RhoA (V14RhoA) with or without FL GDI-1, and SRE reporter activity was determined. SRE-Luc activity is expressed as the ratio of firefly to Renilla luciferase activities. Data represent the mean ± the standard deviation from three experiments performed in triplicate. Asterisks indicate values significantly higher in cells expressing constitutively active RhoA (V14 RhoA) than in cells expressing the vector alone or coexpressing V14RhoA and FL GDI-1 (P < 0.05); +, presence; −, absence.
FIG. 2.
Effect of FL GDI-1 or the C terminus of GDI-1 on thrombin-induced RhoA activity. (A) Autoradiogram showing PKCα-induced phosphorylation of the GDI-1-RhoA complex or the purified GDI-1 protein in vitro. COS-7 cells were cotransduced with the control vector (GFP) or GFP-GDI-1 along with WT RhoA, and after 48 h the lysates were immunoprecipitated with anti-GFP Ab, followed by the addition of protein A/G plus beads. Immunocomplexes, as well as purified GDI-1 protein, were used for in vitro phosphorylation by PKCα as described in Materials and Methods. PKCα induced a greater increase in the phosphorylation of the GDI-1-RhoA complex compared to the purified FL GDI-1 protein. The bottom part of the panel shows the protein loading determined by Western blotting with anti-GDI-1 Ab. An extra band in lane 3 represents the heavy chain of IgG. Data are representative of three independent experiments. (B, top) Autoradiogram showing PKCα phosphorylation of the C terminus but not the N terminus of GDI-1. Purified FL GDI-1 and GDI-1 lacking the C terminus (aa 69 to 204) or the N terminus (aa 1 to 68) were incubated with PKCα in vitro, and phosphorylation was determined as described in Materials and Methods. (B, bottom) Equal protein loading determined by Coomassie blue staining of GDI-1 proteins. (C and D) RhoA activity in response to thrombin in HPAE cells transduced with GFP or GFP-tagged FL GDI-1 or C-GDI-1 mutant. RhoA activity was determined after 2 min of thrombin stimulation. RhoA activation was measured by the increase in the amount of GTP-bound RhoA (C, top) compared to the total amount of RhoA in whole-cell lysates (C, middle). The bottom of panel C shows the expression of GDI-1 mutant proteins determined by Western blotting (WB) with anti-GFP Ab. (D) Plot showing mean values ± standard deviations for the thrombin-induced increase in RhoA activity from multiple experiments calculated as the _n_-fold increase over the basal value under different experimental conditions (n = 3). Asterisks indicate values different from those obtained with unstimulated, vector-expressing cells or cells expressing FL or C-GDI-1 with or without thrombin stimulation (P < 0.05). +, presence; −, absence.
FIG. 3.
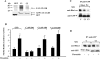
PKCα-induced phosphorylation of the C terminus regulates thrombin-induced SRE generation. (A, top) Autoradiogram showing PKCα-induced phosphorylation of C1- and C2-GDI-1 mutant proteins in vitro. COS-7 cells were transduced with the indicated mutant constructs, and after 48 h the lysates were immunoprecipitated with anti-GFP Ab, followed by addition of protein A/G plus beads. The immunocomplexes were used for in vitro phosphorylation by PKCα as described in Materials and Methods. PKCα induced greater phosphorylation of the C1 domain of GDI-1 compared to the C2 domain. (A, bottom) Western blot assay with anti-GFP Ab indicating equal protein loading. Data are representative of three independent experiments. (B) SRE activity in HPAE cells transduced with the C-, C1-, or C2-GDI-1 mutant construct. HPAE cells were cotransfected with an SRE-luciferase plasmid and GFP or the indicated GFP-tagged C-, C1-, or C2-GDI-1 mutant construct. Cells were then stimulated with thrombin for 5 h prior to SRE activity measurement with the dual-reporter assay kit. SRE-luciferase activity is expressed as the ratio of firefly to Renilla luciferase activities in response to thrombin. Data represent the mean ± the standard deviation from four experiments performed in triplicate. Asterisks indicate values different from those obtained with unstimulated vector-expressing cells or cells expressing the indicated GDI-1 mutant proteins (P < 0.05). +, presence; −, absence. (C) Association of RhoA with C-, C1-, or C2-GDI-1 mutant protein. COS7 cells were cotransfected with equal concentrations of GFP-tagged C-, C1-, or C2-GDI-1 mutant construct along with HA-tagged WT RhoA (RhoA). After 48 h, cell lysates were immunoprecipitated (IP) with anti-GFP Ab, followed by Western blotting (WB) with anti-HA or anti-GFP Abs to determine the association of GDI-1 with RhoA. Data represent results from at least three experiments. The bottom of the panel shows a Western blot assay with anti-GFP Ab indicating mutant protein expression. (D) HPAE cells transduced with the C or C1-GDI-1 mutant construct were stimulated with thrombin for 2 min, and lysates were immunoprecipitated with anti-GFP Ab, followed by Western blotting with anti-RhoA or anti-GFP Abs. Data represent results from at least two experiments. +, presence; −, absence.
FIG. 4.
Effects of nonphosphorylatable truncated GDI-1 mutant proteins on SRE and RhoA activities in response to thrombin. (A) Mutation of Ser96, Ser176, and Thr197 to alanine reduced the phosphorylation of the C1 and C2 domains by PKCα in vitro. COS-7 cells transduced with the indicated mutant constructs were immunoprecipitated with anti-GFP Ab and complexed with protein A/G plus beads. Immunocomplexes were used for in vitro phosphorylation by PKCα as described in Materials and Methods. The top of the panel shows an autoradiograph of a gel, whereas the bottom of the panel shows GFP-tagged protein expression determined by Western blotting with anti-GFP Ab. Data are representative of three independent experiments. (B) HPAE cells cotransfected with the indicated GFP-tagged mutant constructs were stimulated with thrombin for 5 h prior to SRE activity measurement with a dual-reporter assay kit. SRE-luciferase activity is expressed as the ratio of firefly to Renilla luciferase activities quantified as the percent increase in SRE activity over that in unstimulated cells. Data represent the mean ± the standard deviation from three experiments performed in triplicate. The symbols * and # indicate increased SRE activity compared to that obtained with unstimulated cells (*, P < 0.01; #, P < 0.05). (C) HPAE cells transduced with the C1- or S96A-C1-GDI-1 mutant construct were stimulated with 50 nM thrombin for 2 min to determine RhoA activity with GST-bound rhotekin fusion proteins. RhoA activation was measured by the increased amount of GTP-bound RhoA (top) compared to that of RhoA in whole-cell lysates (middle). A Western blot assay with anti-GFP Ab shows mutant protein expression (bottom). (D) Plot showing the mean ± the standard deviation of the thrombin-induced increase in RhoA activity from multiple experiments calculated as the _n_-fold increase over the basal value under various experimental conditions (n = 3). An asterisk indicates an increase in RhoA activity compared to that of a vector-transfected unstimulated monolayer and cells expressing the S96A-C1 mutant protein (P < 0.05). (E) HPAE cells transduced with the C1 or S96A-C1 mutant construct were stimulated with thrombin for 2 min, and lysates were immunoprecipitated (IP) with anti-GFP Ab, followed by Western blotting (WB) with anti-RhoA or anti-GFP Abs. Data represent results from at least two experiments. Symbols in panels B to E: +, presence; −, absence.
FIG. 5.
Phosphorylation of Ser96 is required for SRE and RhoA activation. (A) Mutation of Ser96, Ser176, and Thr197 to alanine markedly reduced the phosphorylation of FL GDI-1 by PKCα in vitro. COS-7 cells transduced with the indicated mutant constructs were immunoprecipitated with anti-GFP Ab and complexed with protein A/G plus beads. Immunocomplexes were used for in vitro phosphorylation by PKCα as described in Materials and Methods. The top of the panel shows the autoradiograph of the gel, whereas the bottom of the panel shows protein expression determined by Western blotting with anti-GFP Ab. Data are representative of three independent experiments. (B) HPAE cells cotransfected with the indicated GFP-tagged mutant proteins were stimulated with thrombin for 5 h prior to SRE activity measurement with a dual-reporter assay kit. SRE-luciferase activity is expressed as the increase in the ratio of firefly to Renilla luciferase activities quantified as an _n_-fold increase in SRE activity over that in unstimulated cells. Data represent the mean ± the standard deviation from three experiments performed in triplicate. An asterisk indicates increased SRE activity compared to that in unstimulated vector-expressing cells or cells expressing the S96A-GDI-1 mutant protein with or without thrombin (P < 0.05). +, presence; −, absence. (C) HPAE cells were cotransfected with the GFP or S96D-GDI-1 mutant construct, and after 24 h, SRE activity was determined with a dual-reporter assay kit. SRE-luciferase activity is expressed as the ratio of firefly to Renilla luciferase activities quantified as the _n_-fold increase in SRE activity over that in GFP-transfected cells. Data represent the mean ± the standard deviation from three experiments performed in triplicate. An asterisk indicates increased SRE activity compared to that in a GFP-transfected monolayer (P < 0.05). (D) HPAE cells transduced with GFP or the S96A- or S96D-GDI-1 mutant construct were stimulated with 50 nM thrombin for 2 min to determine RhoA activity with GST-bound rhotekin fusion proteins. The Western blot assay at the top shows RhoA activity measured by the increased amount of GTP-bound RhoA compared to GFP expression. At the bottom is a plot of the mean ± the standard deviation of the thrombin-induced increase in RhoA activity from multiple experiments calculated as the _n_-fold increase over the basal value under various experimental conditions (n = 3). An asterisk indicates an increase in RhoA activity compared to that in an unstimulated monolayer (P < 0.05). (E and F) Phosphorylation of GDI-1 increased the affinity of GDI-1 for RhoA. (E) HPAE cells transduced with GFP, GFP-tagged FL GDI-1, or the GFP-tagged S96A-, S176A-, T197A-, or S96D-GDI-1 mutant construct were stimulated with thrombin for 2 min, and lysates were immunoprecipitated (IP) with anti-GFP Ab, followed by Western blotting (WB) with anti-RhoA or anti-GFP Ab. Data represent results from at least two experiments. (F) COS7 cells were cotransduced with a GFP or a GFP-tagged S96A- or S96D-GDI-1 mutant construct together with HA-tagged WT RhoA. After 42 h, the lysates were immunoprecipitated with anti-GFP Ab, followed by Western blotting with anti-HA or anti-GFP Ab. Data represent results from at least three experiments. +, presence; −, absence.
FIG. 6.
S96A-GDI-1 mutant protein fails to inhibit Rac1 or Cdc42 activity. (A to D) HPAE cells transduced with the C1-GDI-1 or S96A-C1-GDI-1 mutant construct (A and B) or GFP or the FL S96A-GDI-1 mutant construct (C and D) were stimulated with 50 nM thrombin for the indicated times to determine Rac1 (A and C) or Cdc42 activity (B and D) with GST-bound PAK-binding domain fusion proteins. Rac1 and Cdc42 activation was measured by the increased amount of GTP-bound Rac1 (A and C, top) or GTP-bound Cdc42 (B and D) compared to that of Rac1 or Cdc42 in whole-cell lysates (A to D). A Western blot assay with anti-GFP Ab shows mutant protein expression (A to D, bottom). Data represent results from at least three experiments. The plots in panels A to D show the mean ± standard deviation of thrombin-induced changes in Rac1 or Cdc42 activity from multiple experiments calculated as the _n_-fold increase over the basal value under the indicated experimental conditions (n = 3). An asterisk indicates increased Cdc42 activity in cells expressing the indicated mutant proteins following thrombin stimulation (P < 0.05).
FIG. 7.
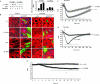
Effects of GDI-1 phosphorylation on MLC phosphorylation, actin stress fiber formation, and increased endothelial permeability in response to thrombin. (A) MLC phosphorylation in response to thrombin in cells transduced with a C1- or C1-S96A-GDI-1 mutant construct. Cells were stimulated with thrombin for the indicated times (indicated in minutes below the lanes), and lysates were Western blotted with anti-phospho-MLC (top) or anti-GFP Abs to determine MLC phosphorylation. The plot shows the mean ± the standard deviation of the thrombin-induced increase in MLC phosphorylation from multiple experiments calculated as the _n_-fold increase over the basal value under the indicated experimental conditions (n = 3). An asterisk indicates increased MLC phosphorylation in cells expressing the indicated mutant proteins following thrombin stimulation (P < 0.05). (B) Actin stress fiber formation in response to thrombin in cells transduced with a GFP-tagged C1-GDI-1, C1-S96A-GDI-1, or FL S96A-GDI-1 mutant construct. Cells were stimulated with thrombin for 5 min, fixed, and then stained with Alexa-labeled phalloidin to determine actin stress fiber formation by confocal imaging. Results are representative of at least four experiments. (C) Cells plated on gold electrodes were transduced with a C1- or C1-S96A-GDI-1 mutant construct to measure the time course of changes in TER after the addition of 50 nM thrombin. Data represent the means ± the standard deviations of changes in TER from multiple experiments. An asterisk indicates a significant decrease in TER in cells expressing C1-S96A-GDI-1 compared to that of cells transducing the C1-GDI-1 mutant protein (P < 0.05). (D) Means ± standard deviations of changes in TER in HPAE cells expressing GFP or the FL S96A-GDI-1 mutant protein. After 30 h posttransfection, cells were stimulated with 50 nM thrombin and changes in TER were monitored. An asterisk indicates a significant decrease in TER in cells expressing the FL S96A-GDI-1 mutant protein compared to that of cells transducing the empty vector (P < 0.05). (E) Cells plated on gold electrodes were transduced with GFP or the FL S96D-GDI-1 mutant construct and after 2 h posttransfection, the time course of changes in TER was determined. Data represent the means ± the standard deviations of changes in TER from multiple experiments. An asterisk indicates a significant increase in TER in cells transduced with the FL S96D-GDI-1 mutant protein compared to that of cells transduced with the empty vector (P < 0.05).
Similar articles
- Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function.
Mehta D, Rahman A, Malik AB. Mehta D, et al. J Biol Chem. 2001 Jun 22;276(25):22614-20. doi: 10.1074/jbc.M101927200. Epub 2001 Apr 17. J Biol Chem. 2001. PMID: 11309397 - Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases.
van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. van Nieuw Amerongen GP, et al. Circ Res. 2000 Aug 18;87(4):335-40. doi: 10.1161/01.res.87.4.335. Circ Res. 2000. PMID: 10948069 - Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA.
Qiao J, Holian O, Lee BS, Huang F, Zhang J, Lum H. Qiao J, et al. Am J Physiol Cell Physiol. 2008 Nov;295(5):C1161-8. doi: 10.1152/ajpcell.00139.2008. Epub 2008 Sep 3. Am J Physiol Cell Physiol. 2008. PMID: 18768928 Free PMC article. - Regulation of RhoA GTPase and various transcription factors in the RhoA pathway.
Kim JG, Islam R, Cho JY, Jeong H, Cap KC, Park Y, Hossain AJ, Park JB. Kim JG, et al. J Cell Physiol. 2018 Sep;233(9):6381-6392. doi: 10.1002/jcp.26487. Epub 2018 Mar 25. J Cell Physiol. 2018. PMID: 29377108 Review. - Vascular endothelial cell activation and permeability responses to thrombin.
Garcia JG, Pavalko FM, Patterson CE. Garcia JG, et al. Blood Coagul Fibrinolysis. 1995 Oct;6(7):609-26. doi: 10.1097/00001721-199510000-00001. Blood Coagul Fibrinolysis. 1995. PMID: 8562832 Review.
Cited by
- Rac-maninoff and Rho-vel: The symphony of Rho-GTPase signaling at excitatory synapses.
Duman JG, Blanco FA, Cronkite CA, Ru Q, Erikson KC, Mulherkar S, Saifullah AB, Firozi K, Tolias KF. Duman JG, et al. Small GTPases. 2022 Jan;13(1):14-47. doi: 10.1080/21541248.2021.1885264. Epub 2021 May 6. Small GTPases. 2022. PMID: 33955328 Free PMC article. - Neural Wiskott-Aldrich syndrome protein (N-WASP)-mediated p120-catenin interaction with Arp2-Actin complex stabilizes endothelial adherens junctions.
Rajput C, Kini V, Smith M, Yazbeck P, Chavez A, Schmidt T, Zhang W, Knezevic N, Komarova Y, Mehta D. Rajput C, et al. J Biol Chem. 2013 Feb 8;288(6):4241-50. doi: 10.1074/jbc.M112.440396. Epub 2012 Dec 4. J Biol Chem. 2013. PMID: 23212915 Free PMC article. - RhoA phosphorylation induces Rac1 release from guanine dissociation inhibitor alpha and stimulation of vascular smooth muscle cell migration.
Rolli-Derkinderen M, Toumaniantz G, Pacaud P, Loirand G. Rolli-Derkinderen M, et al. Mol Cell Biol. 2010 Oct;30(20):4786-96. doi: 10.1128/MCB.00381-10. Epub 2010 Aug 9. Mol Cell Biol. 2010. PMID: 20696841 Free PMC article. - Positive regulation of Rho GTPase activity by RhoGDIs as a result of their direct interaction with GAPs.
Ota T, Maeda M, Okamoto M, Tatsuka M. Ota T, et al. BMC Syst Biol. 2015 Jan 28;9:3. doi: 10.1186/s12918-015-0143-5. BMC Syst Biol. 2015. PMID: 25628036 Free PMC article. - The Andes Virus Nucleocapsid Protein Directs Basal Endothelial Cell Permeability by Activating RhoA.
Gorbunova EE, Simons MJ, Gavrilovskaya IN, Mackow ER. Gorbunova EE, et al. mBio. 2016 Oct 25;7(5):e01747-16. doi: 10.1128/mBio.01747-16. mBio. 2016. PMID: 27795403 Free PMC article.
References
- Chikumi, H., J. Vázquez-Prado, J.-M. Servitja, H. Miyazaki, and J. S. Gutkind. 2002. Potent activation of RhoA by Gαq and Gq-coupled receptors. J. Biol. Chem. 277:27130-27134. - PubMed
- Coughlin, S. R. 2000. Thrombin signalling and protease-activated receptors. Nature 407:258-264. - PubMed
- DerMardirossian, C., and G. M. Bokoch. 2005. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15:356-363. - PubMed
- DerMardirossian, C., A. Schnelzer, and G. M. Bokoch. 2004. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol. Cell 15:117-127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL 84153/HL/NHLBI NIH HHS/United States
- R01 HL071794/HL/NHLBI NIH HHS/United States
- R01 HL084153/HL/NHLBI NIH HHS/United States
- HL 45638/HL/NHLBI NIH HHS/United States
- R01 HL045638/HL/NHLBI NIH HHS/United States
- HL 71794/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous