Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis - PubMed (original) (raw)
Clinical Trial
Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis
Michael F Denny et al. Blood. 2007.
Abstract
Individuals with systemic lupus erythematosus (SLE) have a striking increase in premature atherosclerosis of unclear etiology. Accelerated endothelial cell apoptosis occurs in SLE and correlates with endothelial dysfunction. Endothelial progenitor cells (EPCs) and myelomonocytic circulating angiogenic cells (CACs) are crucial in blood vessel repair after vascular damage, and decreased levels or abnormal function of EPCs/CACs are established atherosclerosis risk factors. We investigated if vascular repair is impaired in SLE. We report that SLE patients display abnormal phenotype and function of EPCs/CACs. These abnormalities are characterized by significant decreases in the number of circulating EPCs (310 +/- 50 EPCs/mL of blood in SLE versus 639 +/- 102 in controls) and significant impairments in the capacity of EPCs/CACs to differentiate into mature ECs and synthesize adequate levels of the proangiogenic molecules vascular endothelial growth factor (VEGF) and hepatic growth factor (HGF). These abnormalities are triggered by interferon-alpha (IFN-alpha), which induces EPC and CAC apoptosis and skews myeloid cells toward nonangiogenic phenotypes. Lupus EPCs/CACs have increased IFN-alpha expression and their supernatants promote higher induction of IFN-inducible genes. Importantly, neutralization of IFN pathways restores a normal EPC/CAC phenotype in lupus. SLE is characterized by an imbalance between endothelial cell damage and repair triggered by type I IFNs, which might promote accelerated atherosclerosis.
Figures
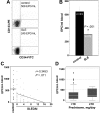
Figure 1
SLE EPCs are decreased in peripheral blood. (A) Dot plots of 1 representative control and 1 representative SLE patient displaying no. of EPCs/mL of blood. Total events for acquisition were as follows: for controls, 127 000 ± 18 000; for SLE, 87 000 ± 6400 (mean ± SEM). (B) Results represent the mean (± SEM). (C) Decreased EPC numbers in SLE correlate with disease activity. EPCs are plotted as a function of SLEDAI score in individual patients. EPC levels in controls also exceed those in lupus patients with SLEDAI scores of zero (639 EPC/mL blood in control vs 370 EPC/mL blood in SLEDAI = 0, P < .05). (D) Lack of association between circulating EPCs and daily prednisone dose in SLE. Error bars are 95% CI whiskers.
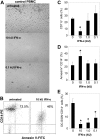
Figure 2
Lupus PBMCs fail to form mature EC monolayers. (A) Control PBMCs were plated into fibronectin-coated wells in complete EC media. On day 15, cells were stained with anti–VWF-FITC, UEA-1–Texas red, and Hoechst 33342 and analyzed by fluorescent microscopy. Images are from 1 representative healthy control and show single fluorophores and a merged image (bottom; × 20 objective magnification). (B) On day 15, wells were examined for EC monolayer development. Representative images of PBMC-derived cells from a healthy control and a lupus patient (× 5 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (C) The y-axis represents the percentage of controls (13/14) or lupus patients (7/33) that formed an EC monolayer.
Figure 3
EC monolayer phenotype in SLE and controls. (A) No differences in acetylated-LDL uptake were detected between SLE and controls, although there were significantly fewer cells in SLE cultures at day 15 (× 10 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (B) Day 15 EC monolayers were analyzed for expression of HLA-DP, -R, and -Q and CD14. Dot plots display a representative healthy control and lupus patient. MHC class II and CD14 expression is consistent with CD14-derived CACs and did not differ between SLE and controls. (C) mRNA expression of proangiogenic factors on day 15 ECs from controls or SLE, normalized to GAPDH. HGF and VEGF-B expression was lower in lupus patients (1-tailed t test, P < .05 for HGF and P = .06 for VEGF-B). Results are mean (± SEM) of 8 patients and 4 controls. (D-E) Proangiogenic molecules are decreased in lupus sera. Results represent mean (± SEM) of 9 controls and 47 SLE patients.
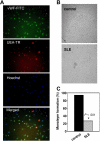
Figure 4
Increased IFN-α expression in lupus EPCs/CACs. (A) Lupus serum prevents monolayer formation by healthy control EPCs/CACs. Control PBMCs were plated on fibronectin-coated wells with complete EC media in the presence or absence of 20% allogeneic control or SLE sera. At day 15, EC monolayer formation was assessed. Images are representative of 7 of 8 SLE serum samples and 4 of 4 control serum samples used in 5 allogeneic control PBMCs. Images represent bright field (top), diI–ac-LDL (middle), and UEA-1–FITC (bottom) of allogeneic control cells exposed to 1 representative SLE serum sample (left) and 1 representative control serum sample (right; × 10 objective magnification). See “In vitro differentiation into mature ECs” for more image acquisition information. (B) Graphs represent percentage IFN-α expression (± SEM) of 13 SLE and 6 controls in PBMCs cultured under proangiogenic conditions. (C) IFN-α expression on day 1–cultured cells of a representative control and SLE patient. (D) EPC/CAC supernatants and autologous serum from SLE patients induce higher expression of IFN-α–inducible genes in epithelial cell lines than controls. Results represent fold induction of IFN-inducible genes (mRNA) and are presented as mean (± SEM) of supernatants or sera from 8 controls and 23 SLE patients. Data are normalized to HPRT-1.
Figure 5
IFN-α treatment prevents EC monolayer formation in healthy controls. (A) Control PBMCs were plated on fibronectin-coated wells with complete EC media in the presence or absence of graded concentrations of recombinant IFN-α. At day 15, EC monolayer formation was assessed. Images are from experiments from 4 representative controls. Similar results were seen in 4 SLE patients using similar concentrations of IFN-α. Magnifications are × 10. See “In vitro differentiation into mature ECs” for more image acquisition information. (B-E) IFN-α induces CAC apoptosis and skewing toward other myeloid cell subsets. Representative dot plots display percentage apoptotic CD14+ CACs in untreated and IFN-α–treated cells. Graphs represent mean (± SEM) percentage expression of myeloid subsets on the EC monolayer of 5 controls after IFN-α treatment. * indicates P < .05.
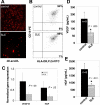
Figure 6
IFN-α induces EPC apoptosis. (A,B) Representative dot plots show increased BM and circulating CD133+ cytotoxicity by IFN-α. (C) Graph is representative of 2 independent experiments from healthy controls.
Figure 7
IFN-α blockade promotes EC monolayer formation in SLE. Lupus PBMCs were cultured under proangiogenic conditions in the presence or absence of (A) blocking antihuman IFN-α mAb or isotype control or (B) antihuman type I IFN-R or isotype control (all 2 μg/mL). At day 15, anti–IFN-α– or anti–type I IFN-R–treated cells, but not controls, formed EC monolayers. Results are representative of independent experiments in SLE patients who failed to form EC monolayers, which corrected with anti–IFN-α (n = 9) or with anti–type I IFN-R (n = 6). (C) TLR7 and/or TLR9 blockade promotes EC monolayer formation in SLE. Lupus PBMCs were cultured in EGM20 supplemented with a control ODN, a TLR7 antagonist, a TLR9 antagonist, or a TLR7/9 antagonist (1 μM). Images were acquired after 15 days in culture. Results show cells from a representative SLE patient. All magnifications are × 20. See “In vitro differentiation into mature ECs” for more image acquisition information.
Similar articles
- The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction.
Thacker SG, Berthier CC, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ. Thacker SG, et al. J Immunol. 2010 Oct 1;185(7):4457-69. doi: 10.4049/jimmunol.1001782. Epub 2010 Aug 30. J Immunol. 2010. PMID: 20805419 Free PMC article. - Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus.
Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Kahlenberg JM, et al. J Immunol. 2011 Dec 1;187(11):6143-56. doi: 10.4049/jimmunol.1101284. Epub 2011 Nov 4. J Immunol. 2011. PMID: 22058412 Free PMC article. - Interleukin 10 hampers endothelial cell differentiation and enhances the effects of interferon α on lupus endothelial cell progenitors.
Cates AM, Holden VI, Myers EM, Smith CK, Kaplan MJ, Kahlenberg JM. Cates AM, et al. Rheumatology (Oxford). 2015 Jun;54(6):1114-23. doi: 10.1093/rheumatology/keu431. Epub 2014 Nov 20. Rheumatology (Oxford). 2015. PMID: 25416712 Free PMC article. - IFN-I Mediates Dysfunction of Endothelial Progenitor Cells in Atherosclerosis of Systemic Lupus Erythematosus.
Ding X, Xiang W, He X. Ding X, et al. Front Immunol. 2020 Nov 11;11:581385. doi: 10.3389/fimmu.2020.581385. eCollection 2020. Front Immunol. 2020. PMID: 33262760 Free PMC article. Review. - Endothelial function and endothelial progenitor cells in systemic lupus erythematosus.
Mak A, Chan JKY. Mak A, et al. Nat Rev Rheumatol. 2022 May;18(5):286-300. doi: 10.1038/s41584-022-00770-y. Epub 2022 Apr 7. Nat Rev Rheumatol. 2022. PMID: 35393604 Review.
Cited by
- Immune mechanisms associated with cardiovascular disease in systemic lupus erythematosus: A path to potential biomarkers.
Guzmán-Martínez G, Marañón C; CYTED RIBLES Network. Guzmán-Martínez G, et al. Front Immunol. 2022 Nov 7;13:974826. doi: 10.3389/fimmu.2022.974826. eCollection 2022. Front Immunol. 2022. PMID: 36420265 Free PMC article. Review. - Endothelial cells: potential novel regulators of renal inflammation.
Oates JC, Russell DL, Van Beusecum JP. Oates JC, et al. Am J Physiol Renal Physiol. 2022 Mar 1;322(3):F309-F321. doi: 10.1152/ajprenal.00371.2021. Epub 2022 Feb 7. Am J Physiol Renal Physiol. 2022. PMID: 35129369 Free PMC article. Review. - An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage.
Kahlenberg JM, Yalavarthi S, Zhao W, Hodgin JB, Reed TJ, Tsuji NM, Kaplan MJ. Kahlenberg JM, et al. Arthritis Rheumatol. 2014 Jan;66(1):152-62. doi: 10.1002/art.38225. Arthritis Rheumatol. 2014. PMID: 24449582 Free PMC article. - The non-haemostatic role of platelets in systemic lupus erythematosus.
Linge P, Fortin PR, Lood C, Bengtsson AA, Boilard E. Linge P, et al. Nat Rev Rheumatol. 2018 Mar 21;14(4):195-213. doi: 10.1038/nrrheum.2018.38. Nat Rev Rheumatol. 2018. PMID: 29559714 Review. - Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus.
Carlucci PM, Purmalek MM, Dey AK, Temesgen-Oyelakin Y, Sakhardande S, Joshi AA, Lerman JB, Fike A, Davis M, Chung JH, Playford MP, Naqi M, Mistry P, Gutierrez-Cruz G, Dell'Orso S, Naz F, Salahuddin T, Natarajan B, Manna Z, Tsai WL, Gupta S, Grayson P, Teague H, Chen MY, Sun HW, Hasni S, Mehta NN, Kaplan MJ. Carlucci PM, et al. JCI Insight. 2018 Apr 19;3(8):e99276. doi: 10.1172/jci.insight.99276. eCollection 2018 Apr 19. JCI Insight. 2018. PMID: 29669944 Free PMC article. Clinical Trial.
References
- Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:338–346. - PubMed
- Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. - PubMed
- Rajagopalan S, Somers EC, Brook RD, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–3683. - PubMed
- Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33:1673–1690. - PubMed
- Luttun A, Carmeliet G, Carmeliet P. Vascular progenitors: from biology to treatment. Trends Cardiovasc Med. 2002;12:88–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA046592/CA/NCI NIH HHS/United States
- P30 CA46592/CA/NCI NIH HHS/United States
- P30 AR48310/AR/NIAMS NIH HHS/United States
- T32-AI07413/AI/NIAID NIH HHS/United States
- R01 HL088419/HL/NHLBI NIH HHS/United States
- P30 AR048310/AR/NIAMS NIH HHS/United States
- K08 AR048235/AR/NIAMS NIH HHS/United States
- AR048235/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical