Human Th2 cells selectively express the orexigenic peptide, pro-melanin-concentrating hormone - PubMed (original) (raw)
Human Th2 cells selectively express the orexigenic peptide, pro-melanin-concentrating hormone
Hilary Sandig et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Th1 and Th2 cells represent the two main functional subsets of CD4(+) T helper cell, and are defined by their cytokine expression. Human Th1 cells express IFNgamma, whilst Th2 cells express IL-4, IL-5, and IL-13. Th1 and Th2 cells have distinct immunological functions, and can drive different immunopathologies. Here, we show that in vitro-differentiated human Th2 cells highly selectively express the gene for pro-melanin-concentrating hormone (PMCH), using real-time RT-PCR, enzyme immunoassay, and Western blot analysis. PMCH encodes the prohormone, promelanin-concentrating hormone (PMCH), which is proteolytically processed to produce several peptides, including the orexigenic hormone melanin-concentrating hormone (MCH). PMCH expression by Th2 cells was activation responsive and increased throughout the 28-day differentiation in parallel with the expression of the Th2 cytokine genes. MCH immunoreactivity was detected in the differentiated Th2 but not Th1 cell culture supernatants after activation, and contained the entire PMCH protein, in addition to several smaller peptides. Human Th1 and Th2 cells were isolated by their expression of IFNgamma and CRTH2, respectively, and the ex vivo Th2 cells expressed PMCH upon activation, in contrast to the Th1 cells. Because Th2 cells are central to the pathogenesis of allergic diseases including asthma, expression of PMCH by activated Th2 cells in vivo may directly link allergic inflammation to energy homeostasis and may contribute to the association between asthma and obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
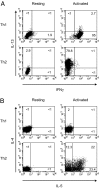
Intracellular cytokine staining of _in vitro_-differentiated Th1 and Th2 cells. Naïve T cells were cultured under either Th1- or Th2-inducing conditions for 28 days, and intracellular cytokine staining was carried out on resting cells or cells that had been activated for 4 h with PMA/ionomycin as indicated, for IL-13 and IFNγ (A) and IL-4 and IL-5 (B). Results shown are representative of six independent experiments.
Fig. 2.
Quantitative RT-PCR on _in vitro_-differentiated Th1 and Th2 cells. (A) Naïve T cells were cultured under either Th1- or Th2-inducing conditions for 28 days. Total RNA was isolated from resting cells (Th1R or Th2R) or cells that had been activated for 4 h with PMA/ionomycin (Th1A or Th2A), and quantitative RT-PCR was carried out with primers to IFNγ, IL-4, IL-5, IL-13, or PMCH. Results are the mean ± SEM of four independent experiments. (B) Total RNA was isolated from resting cells (Th1R or Th2R) or cells that had been activated for 4 h with PMA/ionomycin (Th1A or Th2A) after 14, 21, or 28 days of differentiation, and quantitative RT-PCR was carried out with primers to IFNγ, IL-4, IL-5, IL-13, or PMCH. Results shown are representative of two independent experiments.
Fig. 3.
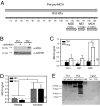
PMCH protein expression by the Th2 cells. (A) Diagram of the PMCH protein, showing the location and length of the peptides, MCH, NEI, and NGE. (B) Cytoplasmic extracts were prepared from resting (−) Th1 and Th2 cells or cells which had been stimulated for 4 h with PMA/ionomycin (+). Western blots were carried out on these extracts with an anti-MCH antibody. Membranes were then reprobed with an antibody to GAPDH. Results shown are representative of three independent experiments. (C) The concentration of MCH in Th1 or Th2 cell culture supernatants (as indicated) was assayed after 14, 21, and 28 days of differentiation. Data shown are the mean ± SEM of three independent experiments. **, P = 0.004 by Tukey two-way ANOVA. (D) After 28 days of differentiation, Th2 cells were removed to fresh media and the concentrations of MCH in supernatants from resting cells, or cells stimulated for 48 or 72 h with plate bound anti-CD3 and anti-CD28 were measured by EIA. By Tukey two-way ANOVA, there is a significant difference between resting and activated cells (*, P = 0.026). (E) An antibody against MCH (α-MCH) or a control antibody (IgG) was used to immunoprecipitate protein from Th1 and Th2 cell culture supernatants or from PBS as indicated. Western blots were carried out on the precipitated material and on untreated supernatant (Input). Data shown are representative of three independent experiments.
Fig. 4.
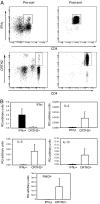
Quantitative RT-PCR on ex vivo Th1 and Th2 cells. (A) CD4+ IFNγ secreting T cells were identified in human PBMCs by using an IFNγ secretion assay, and isolated by FACS. CD4+CRTH2+ T cells were isolated from PBMCs by FACS or with magnetic beads and activated overnight with PMA/ionomycin. (B) Total RNA was isolated, and quantitative RT-PCR was carried out to determine the expression of IFNγ, IL-4, IL-5, IL-13, and PMCH. Data shown are the mean ± SEM from three independent experiments.
Similar articles
- Characterization of melanin concentrating hormone and preproorexin expression in the murine hypothalamus.
Tritos NA, Mastaitis JW, Kokkotou E, Maratos-Flier E. Tritos NA, et al. Brain Res. 2001 Mar 23;895(1-2):160-6. doi: 10.1016/s0006-8993(01)02066-2. Brain Res. 2001. PMID: 11259773 - Chronic loss of melanin-concentrating hormone affects motivational aspects of feeding in the rat.
Mul JD, la Fleur SE, Toonen PW, Afrasiab-Middelman A, Binnekade R, Schetters D, Verheij MM, Sears RM, Homberg JR, Schoffelmeer AN, Adan RA, DiLeone RJ, De Vries TJ, Cuppen E. Mul JD, et al. PLoS One. 2011 May 5;6(5):e19600. doi: 10.1371/journal.pone.0019600. PLoS One. 2011. PMID: 21573180 Free PMC article. - Antisense expression of the human pro-melanin-concentrating hormone genes.
Miller CL, Burmeister M, Thompson RC. Miller CL, et al. Brain Res. 1998 Aug 24;803(1-2):86-94. doi: 10.1016/s0006-8993(98)00626-x. Brain Res. 1998. PMID: 9729295 - Effects of histamine on Th1/Th2 cytokine balance.
Packard KA, Khan MM. Packard KA, et al. Int Immunopharmacol. 2003 Jul;3(7):909-20. doi: 10.1016/S1567-5769(02)00235-7. Int Immunopharmacol. 2003. PMID: 12810348 Review. - [Cytokine measurement at a single-cell level to analyze human Th1 and Th2 cells].
Morinobu A, Kumagai S. Morinobu A, et al. Rinsho Byori. 1998 Sep;46(9):908-14. Rinsho Byori. 1998. PMID: 9800476 Review. Japanese.
Cited by
- Follicular lymphoma cells induce changes in T-cell gene expression and function: potential impact on survival and risk of transformation.
Kiaii S, Clear AJ, Ramsay AG, Davies D, Sangaralingam A, Lee A, Calaminici M, Neuberg DS, Gribben JG. Kiaii S, et al. J Clin Oncol. 2013 Jul 20;31(21):2654-61. doi: 10.1200/JCO.2012.44.2137. Epub 2013 Jun 17. J Clin Oncol. 2013. PMID: 23775959 Free PMC article. - An integrated nano-scale approach to profile miRNAs in limited clinical samples.
Seumois G, Vijayanand P, Eisley CJ, Omran N, Kalinke L, North M, Ganesan AP, Simpson LJ, Hunkapiller N, Moltzahn F, Woodruff PG, Fahy JV, Erle DJ, Djukanovic R, Blelloch R, Ansel KM. Seumois G, et al. Am J Clin Exp Immunol. 2012 Nov 30;1(2):70-89. Am J Clin Exp Immunol. 2012. PMID: 23304658 Free PMC article. - Identification of Prognostic Markers of DNA Damage and Oxidative Stress in Diagnosing Papillary Renal Cell Carcinoma Based on High-Throughput Bioinformatics Screening.
Li L, Liu X, Wen Y, Liu P, Sun T. Li L, et al. J Oncol. 2023 Feb 4;2023:4640563. doi: 10.1155/2023/4640563. eCollection 2023. J Oncol. 2023. PMID: 36785669 Free PMC article. - Melanin-concentrating hormone (MCH) modulates C difficile toxin A-mediated enteritis in mice.
Kokkotou E, Espinoza DO, Torres D, Karagiannides I, Kosteletos S, Savidge T, O'Brien M, Pothoulakis C. Kokkotou E, et al. Gut. 2009 Jan;58(1):34-40. doi: 10.1136/gut.2008.155341. Epub 2008 Sep 29. Gut. 2009. PMID: 18824554 Free PMC article. - IL-4 Causes Hyperpermeability of Vascular Endothelial Cells through Wnt5A Signaling.
Skaria T, Burgener J, Bachli E, Schoedon G. Skaria T, et al. PLoS One. 2016 May 23;11(5):e0156002. doi: 10.1371/journal.pone.0156002. eCollection 2016. PLoS One. 2016. PMID: 27214384 Free PMC article.
References
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. J Immunol. 1986;136:2348–2357. - PubMed
- Abbas AK, Murphy KM, Sher A. Nature. 1996;383:787–793. - PubMed
- Cohn L, Elias JA, Chupp GL. Annu Rev Immunol. 2004;22:789–815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials