The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome - PubMed (original) (raw)
. 2007 Jul 31;104(31):12587-94.
doi: 10.1073/pnas.0705408104. Epub 2007 Jul 18.
Sylvie Dufour, David B Savage, Stefan Bilz, Gina Solomon, Shin Yonemitsu, Gary W Cline, Douglas Befroy, Laura Zemany, Barbara B Kahn, Xenophon Papademetris, Douglas L Rothman, Gerald I Shulman
Affiliations
- PMID: 17640906
- PMCID: PMC1924794
- DOI: 10.1073/pnas.0705408104
The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome
Kitt Falk Petersen et al. Proc Natl Acad Sci U S A. 2007.
Abstract
We examined the hypothesis that insulin resistance in skeletal muscle promotes the development of atherogenic dyslipidemia, associated with the metabolic syndrome, by altering the distribution pattern of postprandial energy storage. Following ingestion of two high carbohydrate mixed meals, net muscle glycogen synthesis was reduced by approximately 60% in young, lean, insulin-resistant subjects compared with a similar cohort of age-weight-body mass index-activity-matched, insulin-sensitive, control subjects. In contrast, hepatic de novo lipogenesis and hepatic triglyceride synthesis were both increased by >2-fold in the insulin-resistant subjects. These changes were associated with a 60% increase in plasma triglyceride concentrations and an approximately 20% reduction in plasma high-density lipoprotein concentrations but no differences in plasma concentrations of TNF-alpha, IL-6, adiponectin, resistin, retinol binding protein-4, or intraabdominal fat volume. These data demonstrate that insulin resistance in skeletal muscle, due to decreased muscle glycogen synthesis, can promote atherogenic dyslipidemia by changing the pattern of ingested carbohydrate away from skeletal muscle glycogen synthesis into hepatic de novo lipogenesis, resulting in an increase in plasma triglyceride concentrations and a reduction in plasma high-density lipoprotein concentrations. Furthermore, insulin resistance in these subjects was independent of changes in the plasma concentrations of TNF-alpha, IL-6, high-molecular-weight adiponectin, resistin, retinol binding protein-4, or intraabdominal obesity, suggesting that these factors do not play a primary role in causing insulin resistance in the early stages of the metabolic syndrome.
Conflict of interest statement
Conflict of interest statement: B.B.K. has a research grant from Takeda Pharmaceuticals and is an inventor on a patent for RBP-4.
Figures
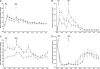
Fig. 1.
Plasma concentrations of glucose (A), insulin (B), triglyceride (C), and fatty acids (D) in insulin-sensitive (○) and insulin-resistant (■) participants before and after the mixed meals.
Fig. 2.
13C MRS measurements of changes in muscle (A) and liver (B) glycogen concentrations and 1H MRS measurements of changes in intramyocellular triglyceride (C) and hepatic triglyceride (D) content in insulin-sensitive and insulin-resistant subjects after the mixed meals.
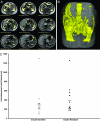
Fig. 3.
MRI imaging of intraabdominal fat of several slices through the abdomen in a subject (A), three-dimensional reconstruction of intraabdominal fat in the same subject (B), and intraabdominal fat volume in the insulin-sensitive and insulin-resistant subjects (C).
Fig. 4.
Fractional de novo lipogenesis in insulin-sensitive and insulin-resistant subjects after the two high-carbohydrate mixed meals.
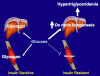
Fig. 5.
Schematic of whole-body energy distribution after high-carbohydrate mixed meals in insulin-sensitive and insulin-resistant individuals.
Similar articles
- Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals.
Rabøl R, Petersen KF, Dufour S, Flannery C, Shulman GI. Rabøl R, et al. Proc Natl Acad Sci U S A. 2011 Aug 16;108(33):13705-9. doi: 10.1073/pnas.1110105108. Epub 2011 Aug 1. Proc Natl Acad Sci U S A. 2011. PMID: 21808028 Free PMC article. Clinical Trial. - Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly.
Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF. Flannery C, et al. Diabetes. 2012 Nov;61(11):2711-7. doi: 10.2337/db12-0206. Epub 2012 Jul 24. Diabetes. 2012. PMID: 22829450 Free PMC article. - The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome.
Jornayvaz FR, Samuel VT, Shulman GI. Jornayvaz FR, et al. Annu Rev Nutr. 2010 Aug 21;30:273-90. doi: 10.1146/annurev.nutr.012809.104726. Annu Rev Nutr. 2010. PMID: 20645852 Free PMC article. Review. - Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome.
Belfort R, Berria R, Cornell J, Cusi K. Belfort R, et al. J Clin Endocrinol Metab. 2010 Feb;95(2):829-36. doi: 10.1210/jc.2009-1487. Epub 2010 Jan 8. J Clin Endocrinol Metab. 2010. PMID: 20061429 Free PMC article. Clinical Trial.
Cited by
- High-fat-diet-induced hepatic insulin resistance per se attenuates murine de novo lipogenesis.
Goedeke L, Strober JW, Suh R, Paolella LM, Li X, Rogers JC, Petersen MC, Nasiri AR, Casals G, Kahn M, Cline GW, Samuel VT, Shulman GI, Vatner DF. Goedeke L, et al. iScience. 2024 Oct 15;27(11):111175. doi: 10.1016/j.isci.2024.111175. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524330 Free PMC article. - Drivers of cardiovascular disease in metabolic dysfunction-associated steatotic liver disease: the threats of oxidative stress.
Minetti ET, Hamburg NM, Matsui R. Minetti ET, et al. Front Cardiovasc Med. 2024 Oct 1;11:1469492. doi: 10.3389/fcvm.2024.1469492. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39411175 Free PMC article. Review. - Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy.
Lu X, Xie Q, Pan X, Zhang R, Zhang X, Peng G, Zhang Y, Shen S, Tong N. Lu X, et al. Signal Transduct Target Ther. 2024 Oct 2;9(1):262. doi: 10.1038/s41392-024-01951-9. Signal Transduct Target Ther. 2024. PMID: 39353925 Free PMC article. Review. - The additive effect of the triglyceride-glucose index and estimated glucose disposal rate on long-term mortality among individuals with and without diabetes: a population-based study.
He HM, Xie YY, Chen Q, Li YK, Li XX, Mu YK, Duo XY, Gao YX, Zheng JG. He HM, et al. Cardiovasc Diabetol. 2024 Aug 22;23(1):307. doi: 10.1186/s12933-024-02396-8. Cardiovasc Diabetol. 2024. PMID: 39175051 Free PMC article. - Small molecule inhibition of glycogen synthase I reduces muscle glycogen content and improves biomarkers in a mouse model of Pompe disease.
Gaspar RC, Sakuma I, Nasiri A, Hubbard BT, LaMoia TE, Leitner BP, Tep S, Xi Y, Green EM, Ullman JC, Petersen KF, Shulman GI. Gaspar RC, et al. Am J Physiol Endocrinol Metab. 2024 Oct 1;327(4):E524-E532. doi: 10.1152/ajpendo.00175.2024. Epub 2024 Aug 22. Am J Physiol Endocrinol Metab. 2024. PMID: 39171753
References
- Reaven GM. Diabetes. 1988;37:1595–1607. - PubMed
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Circulation. 2004;109:433–438. - PubMed
- Previs SF, Hazey JW, Diraison F, Beylot M, David F, Brunengraber H. J Mass Spectrom. 1996;31:639–642. - PubMed
- Diraison F, Pachiaudi C, Beylot M. J Mass Spectrom. 1997;32:81–86. - PubMed
- Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, Kissebah AH. Ann Intern Med. 1989;110:867–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR 00125/RR/NCRR NIH HHS/United States
- R01 EB006494/EB/NIBIB NIH HHS/United States
- P01 DK068229/DK/NIDDK NIH HHS/United States
- P01 DK 068229/DK/NIDDK NIH HHS/United States
- R01 DK 43051/DK/NIDDK NIH HHS/United States
- R01 AG023686/AG/NIA NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R01 DK043051/DK/NIDDK NIH HHS/United States
- R01 EB 006494/EB/NIBIB NIH HHS/United States
- R01 AG 23686/AG/NIA NIH HHS/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- P30 DK 45735/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical