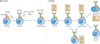B-cell anergy: from transgenic models to naturally occurring anergic B cells? - PubMed (original) (raw)
Review
B-cell anergy: from transgenic models to naturally occurring anergic B cells?
John C Cambier et al. Nat Rev Immunol. 2007 Aug.
Abstract
Anergy, a condition in which cells persist in the periphery but are unresponsive to antigen, is responsible for silencing many self-reactive B cells. Loss of anergy is known to contribute to the development of autoimmune diseases, including systemic lupus erythematosus and type 1 diabetes. Multiple transgenic mouse models have enabled the dissection of mechanisms that underlie anergy, and recently, anergic B cells have been identified in the periphery of wild-type mice. Heterogeneity of mechanistic concepts developed using model systems has complicated our understanding of anergy and its biological features. In this Review, we compare and contrast the salient features of anergic B cells with a view to developing unifying mechanistic hypotheses that explain their lifestyles.
Figures
Figure 1. Stages of B-cell development
B-cell development occurs in both the bone marrow and peripheral lymphoid tissues such as the spleen. In the bone marrow, development progresses through the pro-B-cell, pre-B-cell and immature-B-cell stages. During this differentiation, rearrangements at the immunoglobulin locus result in the generation and surface expression of the pre-B-cell receptor (pre-BCR, which is comprised of an Igµ heavy chain and surrogate light chains (VpreB or Vλ5)) and finally a mature BCR (comprised of rearranged heavy- and light-chain genes) that is capable of binding antigen. At this immature stage of development, B cells undergo a selection process to prevent any further development of self-reactive cells. Both receptor editing and clonal deletion have a role at this stage. Cells successfully completing this checkpoint leave the bone marrow as transitional B cells, eventually maturing into mature follicular B cells (or marginal-zone B cells). Following an immune response, antigen-specific B cells develop into either plasma (antibody-secreting) cells or memory B cells. Transitional 3 (T3) B cells, once thought to be part of the linear development from immature to mature B cells, are now thought to represent primarily self-reactive anergic B cells (also known as An1 B cells).
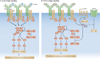
Figure 2. B-cell signalling in response to acute or chronic antigen stimulation
a | The normal B-cell receptor (BCR) response to acute antigen stimulation involves the phosphorylation of two tyrosine residues in the ITAMs (immunoreceptor tyrosine-based activation motifs) of Igα and Igβ by LYN or other SRC-family kinases. This results in the recruitment and activation of SYK (spleen tyrosine kinase), which in turn phosphorylates key downstream substrates such as BLNK (B-cell linker) and BTK (Bruton’s tyrosine kinase), resulting in productive BCR signalling, activation and productive interactions with helper T cells. b | In anergic B cells, chronic binding of self antigen is thought to favour monophosphorylation of ITAM tyrosines. This limits the recruitment and activation of SYK, yet allows efficient LYN activity, which enhances the phosphorylation and activation of an inhibitory complex containing SHIP1 (SRC-homology-2-domain-containing inositol-5-phosphatase 1) and DOK (docking protein). These proteins act to dampen any productive BCR-mediated signalling in a manner that is analogous to FcγRIIb (an inhibitory Fc receptor for IgG that is expressed by B cells). This inhibitory complex may also act in trans to inhibit signalling by other receptors, including CXC-chemokine receptor 4 (CXCR4) and possibly the receptor for B-cell-activating factor (BAFFR). DAG, diacylglycerol; ERK, extracellular-signal-regulated kinase; GRB2, growth-factor-receptor-bound protein 2; InsP3, inositol-1,4,5-trisphosphate; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PLCγ, phospholipase Cγ; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; SOS, son-of-sevenless homologue.
Similar articles
- Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling.
Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Gauld SB, et al. Nat Immunol. 2005 Nov;6(11):1160-7. doi: 10.1038/ni1256. Epub 2005 Oct 2. Nat Immunol. 2005. PMID: 16200069 - Identification of anergic B cells within a wild-type repertoire.
Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Merrell KT, et al. Immunity. 2006 Dec;25(6):953-62. doi: 10.1016/j.immuni.2006.10.017. Immunity. 2006. PMID: 17174121 - B-cell anergy is maintained in anti-DNA transgenic NZB/NZW mice.
Kat I, Makdasi E, Fischel R, Eilat D. Kat I, et al. Int Immunol. 2010 Feb;22(2):101-11. doi: 10.1093/intimm/dxp120. Epub 2009 Dec 27. Int Immunol. 2010. PMID: 20038519 - Silencing of autoreactive B cells by anergy: a fresh perspective.
Gauld SB, Merrell KT, Cambier JC. Gauld SB, et al. Curr Opin Immunol. 2006 Jun;18(3):292-7. doi: 10.1016/j.coi.2006.03.015. Epub 2006 Apr 17. Curr Opin Immunol. 2006. PMID: 16616480 Review. - Autoreactivity and the positive selection of B cells.
Übelhart R, Jumaa H. Übelhart R, et al. Eur J Immunol. 2015 Nov;45(11):2971-7. doi: 10.1002/eji.201444622. Epub 2015 Sep 9. Eur J Immunol. 2015. PMID: 26350303 Review.
Cited by
- Polygenic autoimmune disease risk alleles impacting B cell tolerance act in concert across shared molecular networks in mouse and in humans.
Harley ITW, Allison K, Scofield RH. Harley ITW, et al. Front Immunol. 2022 Aug 24;13:953439. doi: 10.3389/fimmu.2022.953439. eCollection 2022. Front Immunol. 2022. PMID: 36090990 Free PMC article. Review. - Role of molecular mimicry and polyclonal cell activation in the induction of pathogenic β2-glycoprotein I-directed immune response in Balb/c mice upon hyperimmunization with tetanus toxoid.
Stojanović M, Petrušić V, Zivković I, Inić-Kanada A, Stojićević I, Marinković E, Dimitrijević L. Stojanović M, et al. Immunol Res. 2013 May;56(1):20-31. doi: 10.1007/s12026-012-8343-1. Immunol Res. 2013. PMID: 22875539 - Endogenous antigen tunes the responsiveness of naive B cells but not T cells.
Zikherman J, Parameswaran R, Weiss A. Zikherman J, et al. Nature. 2012 Sep 6;489(7414):160-4. doi: 10.1038/nature11311. Nature. 2012. PMID: 22902503 Free PMC article. - Self-reactivity on a spectrum: A sliding scale of peripheral B cell tolerance.
Tan C, Noviski M, Huizar J, Zikherman J. Tan C, et al. Immunol Rev. 2019 Nov;292(1):37-60. doi: 10.1111/imr.12818. Epub 2019 Oct 20. Immunol Rev. 2019. PMID: 31631352 Free PMC article. Review. - Pten controls B-cell responsiveness and germinal center reaction by regulating the expression of IgD BCR.
Setz CS, Khadour A, Renna V, Iype J, Gentner E, He X, Datta M, Young M, Nitschke L, Wienands J, Maity PC, Reth M, Jumaa H. Setz CS, et al. EMBO J. 2019 Jun 3;38(11):e100249. doi: 10.15252/embj.2018100249. Epub 2019 Apr 23. EMBO J. 2019. PMID: 31015337 Free PMC article.
References
- Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. This paper shows that a high frequency of early immature B cells generated during human B-cell development are self-reactive.
- Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. - PubMed
- Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources