Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR - PubMed (original) (raw)
Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR
Beatrice Vitali et al. Appl Environ Microbiol. 2007 Sep.
Abstract
The microbial flora of the vagina plays a major role in preventing genital infections, including bacterial vaginosis (BV) and candidiasis (CA). An integrated approach based on PCR-denaturing gradient gel electrophoresis (PCR-DGGE) and real-time PCR was used to study the structure and dynamics of bacterial communities in vaginal fluids of healthy women and patients developing BV and CA. Universal eubacterial primers and Lactobacillus genus-specific primers, both targeted at 16S rRNA genes, were used in DGGE and real-time PCR analysis, respectively. The DGGE profiles revealed that the vaginal flora was dominated by Lactobacillus species under healthy conditions, whereas several potentially pathogenic bacteria were present in the flora of women with BV. Lactobacilli were the predominant bacterial population in the vagina for patients affected by CA, but changes in the composition of Lactobacillus species were observed. Real-time PCR analysis allowed the quantitative estimation of variations in lactobacilli associated with BV and CA diseases. A statistically significant decrease in the relative abundance of lactobacilli was found in vaginal fluids of patients with BV compared to the relative abundance of lactobacilli in the vaginal fluids of healthy women and patients with CA.
Figures
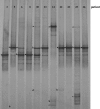
FIG. 1.
DGGE profiles of healthy women (NI group). A single DGGE profile was reported for each woman in the NI group. The bands correspond to bacterial taxa and GenBank accession numbers as follows: Lactobacillus iners, 3a (AB279893), 6b (AB279894), 8a (AB279895), and 10c (AB279896); Lactobacillus sp., 5a (AB279907) and 26a (AB279908); Lactobacillus acidophilus, 6a (AB279917), 10b (AB279918), 11a (AB279919), 22a (AB279920), 23a (AB279921), and 25b (AB279922); Lactobacillus gasseri, 10a (AB279926), 14a (AB279927), and 25a (AB279928); Lactobacillus vaginalis, 10d (AB279933) and 22b (AB279934); Gardnerella vaginalis, 5b (AB279938), 14c (AB279939), and 25c (AB279940); uncultured Gardnerella sp., 14b (AB279952).
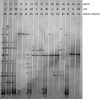
FIG. 2.
DGGE profiles of patients developing BV. Three DGGE courses corresponding to the three monthly gynecologic visits were reported. The bands correspond to bacterial taxa and GenBank accession numbers as follows: Lactobacillus iners, 17b (AB279897), 20e (AB279898), and 24b (AB279899); Lactobacillus acidophilus, 17a (AB279923) and 20d (AB279924); Lactobacillus plantarum, 20a (AB279937); Lactobacillus gasseri, 12a (AB279929) and 20b (AB279930); Lactobacillus vaginalis, 17c (AB279935); Atopobium vaginae, 2q (AB279953) and 12g (AB279954); Gardnerella vaginalis, 2m (AB279941), 2o (AB279942), 2p (AB279943), 17d (AB279944), 17e (AB279945), and 24c (AB279946); Leptotrichia amnionii, 2a (AB279955), 2b (AB279956), and 2d (AB279957); Leptotrichia sanguinegens, 24a (AB279960); uncultured Prevotella sp., 2c (AB279962), 2f (AB279963), 2g (AB279964), and 2h (AB279965); Prevotella sp., 12b (AB279968) and 12e (AB279969); uncultured Megasphaera sp., 2l (AB279970), 20f (AB279971), and 20g (AB279972); uncultured Chloroflexi bacterium, 2i (AB279973); uncultured Clostridium sp., 2n (AB279974); Streptococcus sp., 12c (AB279977); Staphylococcus sp., 2e (AB279978) and 20c (AB279979); Veillonella montpellierensis, 12d (AB279980) and 12f (AB279981).
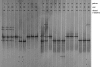
FIG. 3.
DGGE profiles of patients developing CA. Three DGGE courses corresponding to the three monthly gynecologic visits were reported. The bands correspond to bacterial taxa and GenBank accession numbers as follows: Lactobacillus sp., 1a (AB279909), 1b (AB279910), 1c (AB279911), 1d (AB279912), 4b (AB279913), 13a (AB279914), and 19a (AB279915); Lactobacillus iners, 4c (AB279900), 16c (AB279901), 18a (AB279902), and 21b (AB279903); Lactobacillus gassseri, 16a (AB279931) and 21a (AB279932); Lactobacillus acidophilus, 16b (AB279925); Lactobacillus vaginalis, 4d (AB279936); Gardnerella vaginalis, 21c (AB279947) and 21d (AB279948); uncultured Ureaplasma sp., 4a (AB279982); uncultured Clostridium sp., 4e (AB279976); Shigella boydii, 18b (AB279983).
FIG. 4.
DGGE profiles of patients developing both BV and CV (BV-CA). Three DGGE courses corresponding to the three monthly gynecologic visits were reported. The bands correspond to bacterial taxa and GenBank accession numbers as follows: Lactobacillus iners, 7e (AB279904), 9a (AB279905), and 15b (AB279906); Lactobacillus sp., 15a (AB279916); Gardnerella vaginalis, 7h (AB279949), 7i (AB279950), and 9b (AB279951); Leptotrichia amnionii, 7a (AB279958) and 7b (AB279959); Leptotrichia sanguinegens, 7c (AB279961); uncultured Prevotella sp., 7d (AB279966) and 7f (AB279967); uncultured Clostridium sp., 7g (AB279975).
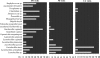
FIG. 5.
Frequency of the occurrence of bacterial species in relation to clinical status of the subject. Frequencies were calculated as the ratios (percentage) of the number of visits characterized by the presence of each species to the total number of visits belonging to each visit group (NI, BV, and CA).
FIG. 6.
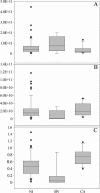
Real-time PCR evaluation of 16S rrn operons of total eubacteria (A) and lactobacilli (B) and relative abundance of lactobacilli (C) related to each group of visits characterized by a specific clinical status (NI, BV, and CA). The number of operons in 1 ml of vaginal rinsing is expressed as an absolute value. Lactobacillus relative abundance is expressed as the ratio of lactobacillus rrn operons to total eubacterial 16S rrn operons. The box for each group represents the interquartile range (25th to 75th percentile), and the line within this box is the median value. The bottom and top bars indicate the 10th and 90th percentiles, respectively. Outlier values are indicated (black circles).
FIG. 7.
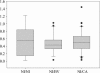
Relative abundances of lactobacilli in the vaginal flora of women during NI visits, relative to the clinical history of the women. Lactobacillus relative abundance is expressed as the ratio of lactobacillus 16S rrn operons to total eubacterial 16S rrn operons. The box for each group represents the interquartile range (25th to 75th percentile), and the line within this box is the median value. The bottom and top bars indicate the 10th and 90th percentiles, respectively. Outlier values are indicated (black circles).
Similar articles
- Organism diversity between women with and without bacterial vaginosis as determined by polymerase chain reaction denaturing gradient gel electrophoresis and 16S rRNA gene sequence.
Diao Y, Fang X, Xia Q, Chen S, Li H, Yang Y, Wang Y, Li H, Cui J, Sun X, Zhao Z. Diao Y, et al. J Obstet Gynaecol Res. 2011 Oct;37(10):1438-46. doi: 10.1111/j.1447-0756.2011.01564.x. Epub 2011 Jun 16. J Obstet Gynaecol Res. 2011. PMID: 21676075 - Complexity of vaginal microflora as analyzed by PCR denaturing gradient gel electrophoresis in a patient with recurrent bacterial vaginosis.
Devillard E, Burton JP, Reid G. Devillard E, et al. Infect Dis Obstet Gynecol. 2005 Mar;13(1):25-31. doi: 10.1080/10647440400025504. Infect Dis Obstet Gynecol. 2005. PMID: 16040324 Free PMC article. - Vaginal microbiome.
Buchta V. Buchta V. Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English. - Characterization of the vaginal microflora in health and disease.
Datcu R. Datcu R. Dan Med J. 2014 Apr;61(4):B4830. Dan Med J. 2014. PMID: 24814599 Review.
Cited by
- The effect of antifungal treatment on the vaginal flora of women with vulvo-vaginal yeast infection with or without bacterial vaginosis.
Donders G, Bellen G, Ausma J, Verguts L, Vaneldere J, Hinoul P, Borgers M, Janssens D. Donders G, et al. Eur J Clin Microbiol Infect Dis. 2011 Jan;30(1):59-63. doi: 10.1007/s10096-010-1052-6. Epub 2010 Sep 28. Eur J Clin Microbiol Infect Dis. 2011. PMID: 20878199 - Vaginal microbiota of women with frequent vulvovaginal candidiasis.
Zhou X, Westman R, Hickey R, Hansmann MA, Kennedy C, Osborn TW, Forney LJ. Zhou X, et al. Infect Immun. 2009 Sep;77(9):4130-5. doi: 10.1128/IAI.00436-09. Epub 2009 Jun 15. Infect Immun. 2009. PMID: 19528218 Free PMC article. - The vaginal microbiome: new information about genital tract flora using molecular based techniques.
Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R. Lamont RF, et al. BJOG. 2011 Apr;118(5):533-49. doi: 10.1111/j.1471-0528.2010.02840.x. Epub 2011 Jan 20. BJOG. 2011. PMID: 21251190 Free PMC article. Review. - Vaginal microbiota of healthy pregnant Mexican women is constituted by four Lactobacillus species and several vaginosis-associated bacteria.
Hernández-Rodríguez C, Romero-González R, Albani-Campanario M, Figueroa-Damián R, Meraz-Cruz N, Hernández-Guerrero C. Hernández-Rodríguez C, et al. Infect Dis Obstet Gynecol. 2011;2011:851485. doi: 10.1155/2011/851485. Epub 2011 Sep 22. Infect Dis Obstet Gynecol. 2011. PMID: 21960733 Free PMC article. - Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis.
Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, Xia Y, Xiang C. Ling Z, et al. BMC Genomics. 2010 Sep 7;11:488. doi: 10.1186/1471-2164-11-488. BMC Genomics. 2010. PMID: 20819230 Free PMC article.
References
- Altermann, E., W. M. Russell, M. A. Azcarate-Peril, R. Barrangou, B. L. Buck, O. McAuliffe, N. Souther, A. Dobson, T. Duong, M. Callanan, S. Lick, A. Hamrick, R. Cano, and T. R. Klaenhammer. 2005. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 102:3906-3912. - PMC - PubMed
- Amsel, R., P. A. Totten, C. A. Spiegel, C. K. Chen, D. Eschenbach, and K. K. Holmes. 1983. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiological associations. Am. J. Med. 74:14-22. - PubMed
- Antonio, M. A., S. E. Hawes, and S. L. Hillier. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 180:1950-1956. - PubMed
- Aroutcheva, A., D. Gariti, M. Simon, S. Shott, J. Faro, J. A. Simoes, A. Gurguis, and S. Faro. 2001. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 185:375-379. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical