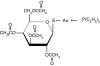Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3 - PubMed (original) (raw)
Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3
Nam-Hoon Kim et al. Immunology. 2007 Dec.
Abstract
Auranofin (AF) is a sulphur-containing gold compound. Because of its anti-inflammatory and immunosuppressive activities, AF has been widely used for the therapeutic treatment of rheumatoid arthritis. However, little is known about its mechanism of action. To elucidate the molecular mechanism underlying the anti-inflammatory effect of AF, we studied the effects of AF on cellular responses to interleukin-6 (IL-6). In HepG2 human hepatoma cells, AF markedly inhibited IL-6-induced phosphorylation of janus kinase 1 (JAK1) and signal transducer and activator of transcription 3 (STAT3) and STAT3 translocation into the nucleus. Consistent with this, AF diminished IL-6-induced production of the acute-phase proteins, haptoglobin, fibrinogen, C3 complement and alpha(1)-acid glycoprotein, and gene expression of vascular endothelial growth factor, all of whose transcriptional activities are regulated by STAT3. The inhibitory activity of AF on STAT3 phosphorylation was also demonstrated in primary cells, i.e. fibroblast-like synoviocytes from rheumatoid arthritis patients, human umbilical vein endothelial cells and rat astrocytes. Auranofin-mediated inhibition of STAT3 phosphorylation was recovered by pretreatment with antioxidants containing thiol groups. These findings suggest that the anti-inflammatory action of AF is associated with a blockade of JAK1/STAT3 signalling. Thiol-group-reactive proteins may be involved in AF-induced suppression of JAK1/STAT3 phosphorylation.
Figures
Figure 1
The chemical structure of auranofin.
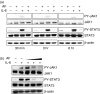
Figure 2
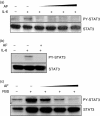
The effect of AF on IL-6-induced JAK1/STAT3 phosphorylation. (a) HepG2 cells were pretreated with 2 μ
m
AF for 30 min and then incubated with IL-6 (10 ng/ml) for 30 min, 3 hr, or 6 hr. At the indicated times, cellular proteins were extracted and analysed by Western blotting using antibodies against JAK1, STAT3, PY-JAK1, PY-STAT3, and β-actin. (b) HepG2 cells were preincubated with AF (1, 2 and 3 μ
m
) for 30 min and then incubated with IL-6 (10 ng/ml) for 6 hr.
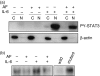
Figure 3
The inhibitory effect of AF on translocation of STAT3 to the nucleus. HepG2 cells were preincubated without or with 2 µ
m
AF for 30 min and then stimulated with IL-6 (10 ng/ml) for 30 min (a) Cell lysates were subfractionated into cytoplasmic (C) and nuclear (N) fractions. PY-STAT3 distribution was determined by Western blotting. The data for β-actin, an internal marker for the cytoplasmic fraction, show that the fractionation was successful. (b) Nuclear extracts (2 μg protein) were incubated with 32P-labelled oligonucleotides containing STAT3-binding consensus sequences for 15 min at room temperature. The samples were then separated on 7% polyacrylamide gel and visualized by autoradiography. To verify the specificity of binding, competition experiments were carried out using unlabelled wild and mutant oligonucleotides for STAT3 binding as described in the materials and methods section. Note that the unlabelled wild-type oligonucleotide, but not the mutant oligonucleotide, completely inhibited binding of the probe, suggesting that STAT3 binding is specific.
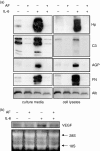
Figure 4
Down-regulation of gene expression of acute-phase proteins (a) and VEGF (b). AF-pretreated HepG2 cells were stimulated with IL-6 (10 ng/ml). (a) After 24 hr of stimulation, various acute-phase proteins in culture media (secreted proteins) and cell lysates were analysed by Western blotting using antibodies against Hp, C3 complement (C3), α1-acid glycoprotein (AGP), fibrinogen (FN), and albumin (Alb). (b) After 12-hr stimulation, total cellular RNAs were extracted and analysed by Northern blotting using a human VEGF probe. Ethidium-bromide-stained 18S and 28S ribosomal RNAs were used as internal markers to ensure equal RNA loading.
Figure 5
Inhibition of STAT3 phosphorylation by AF in primary cells associated with inflammation. (a) FLS prepared from patients with rheumatoid arthritis were incubated for 24 hr in serum-free DMEM. The serum-starved cells were pretreated with AF (0·1, 0·3, 0·5 and 1 µ
m
) for 30 min and then incubated with IL-6 (20 ng/ml) for 30 min. The cell lysates were analysed by Western blotting using anti-STAT3 and anti-PY-STAT3 antibodies. (b) Fresh astrocytes purified from rat brain were preincubated without or with AF (0·2 µ
m
) for 30 min and the cells were stimulated with IL-6 (10 ng/ml) for 1 hr. (c) HUVECs were prepared from a fresh umbilical cord and serum-starved for 8 hr in M199 medium containing 0·5% FBS. After incubation for 30 min in AF (0·1, 0·3, and 0·5 μ
m
), the cells were stimulated with 10% FBS for 30 min. The results shown here are representative of three separate experiments using three independently isolated FLS (a), astrocytes (b) and HUVECs (c).
Figure 6
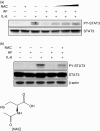
Recovery of AF-mediated inhibition of STAT3 phosphorylation by NAC. (a) HepG2 cells were preincubated with 0·5, 1, and 5 m
m
NAC for 30 min and stimulated with IL-6 (10 ng/ml) in the absence or presence of AF (2 μ
m
). After stimulation for 1 hr, the cells were lysed and analysed by Western blotting using anti-STAT3 and anti-PY-STAT3 antibodies. (b) Fresh astrocytes isolated from rat brain were preincubated with 10 m
m
NAC for 30 min and stimulated with IL-6 (10 ng/ml) in the absence or presence of AF (0·2 μ
m
) for 1 hr.
Figure 7
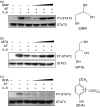
Thiol-dependent recovery of AF-inhibited STAT3 phosphorylation. HepG2 cells were preincubated for 30 min with antioxidants containing thiol groups (dimercaptopropanol; DMP and monothioglycerol; MTG) or an antioxidant that did not contain a thiol group (butylated hydroxyanisole; BHA) before exposure to 2 μ
m
AF. After preincubation, the cells were treated with AF and stimulated with IL-6 (10 ng/ml). The cell lysates were analysed by Western blotting. (a) (b) and (c) correspond to pretreatment with dimercaptopropanol (100, 250 and 500 µ
m
), monothioglycerol (1, 5 and 10 m
m
), and butylated hydroxyanisole (50, 100 and 250 μ
m
), respectively.
Similar articles
- Chrysin suppresses IL-6-induced angiogenesis via down-regulation of JAK1/STAT3 and VEGF: an in vitro and in ovo approach.
Lin CM, Shyu KG, Wang BW, Chang H, Chen YH, Chiu JH. Lin CM, et al. J Agric Food Chem. 2010 Jun 9;58(11):7082-7. doi: 10.1021/jf100421w. J Agric Food Chem. 2010. PMID: 20443595 - The gold compound auranofin induces apoptosis of human multiple myeloma cells through both down-regulation of STAT3 and inhibition of NF-κB activity.
Nakaya A, Sagawa M, Muto A, Uchida H, Ikeda Y, Kizaki M. Nakaya A, et al. Leuk Res. 2011 Feb;35(2):243-9. doi: 10.1016/j.leukres.2010.05.011. Epub 2010 Jun 12. Leuk Res. 2011. PMID: 20542334 - [Mechanism of action of gold compounds].
Vischer TL. Vischer TL. Wien Klin Wochenschr Suppl. 1984;156:17-8. Wien Klin Wochenschr Suppl. 1984. PMID: 6442050 Review. German. - Glioblastoma invasion, cathepsin B, and the potential for both to be inhibited by auranofin, an old anti-rheumatoid arthritis drug.
Kast RE. Kast RE. Cent Eur Neurosurg. 2010 Aug;71(3):139-42. doi: 10.1055/s-0029-1242756. Epub 2010 Feb 1. Cent Eur Neurosurg. 2010. PMID: 20127591 Review. No abstract available.
Cited by
- GOLD-Induced Cytokine (GOLDIC): A Critical Review of Its Properties, Synthesis, and Biomedical Applications.
Yadav S, Rawal G, Jeyaraman N, Jeyaraman M. Yadav S, et al. Cureus. 2024 Jan 11;16(1):e52130. doi: 10.7759/cureus.52130. eCollection 2024 Jan. Cureus. 2024. PMID: 38344607 Free PMC article. Review. - The tumor proteasome as a novel target for gold(III) complexes: implications for breast cancer therapy.
Milacic V, Dou QP. Milacic V, et al. Coord Chem Rev. 2009;253(11-12):1649-1660. doi: 10.1016/j.ccr.2009.01.032. Coord Chem Rev. 2009. PMID: 20047011 Free PMC article. - Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship?
Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Aggarwal BB, et al. Ann N Y Acad Sci. 2009 Aug;1171:59-76. doi: 10.1111/j.1749-6632.2009.04911.x. Ann N Y Acad Sci. 2009. PMID: 19723038 Free PMC article. Review. - Programmable melanoma-targeted radio-immunotherapy via fusogenic liposomes functionalized with multivariate-gated aptamer assemblies.
Ren X, Xue R, Luo Y, Wang S, Ge X, Yao X, Li L, Min J, Li M, Luo Z, Wang F. Ren X, et al. Nat Commun. 2024 Jun 12;15(1):5035. doi: 10.1038/s41467-024-49482-9. Nat Commun. 2024. PMID: 38866788 Free PMC article. - Auranofin Suppresses Plasminogen Activator Inhibitor-2 Expression through Annexin A5 Induction in Human Prostate Cancer Cells.
Shin DW, Kwon YJ, Ye DJ, Baek HS, Lee JE, Chun YJ. Shin DW, et al. Biomol Ther (Seoul). 2017 Mar 1;25(2):177-185. doi: 10.4062/biomolther.2016.223. Biomol Ther (Seoul). 2017. PMID: 27956714 Free PMC article.
References
- Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–6. - PubMed
- Hirano T. Interleukin 6 and its receptor. Ten years later. Int Rev Immunol. 1998;16:249–84. - PubMed
- Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–68. - PubMed
- Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:277–81. - PubMed
- Yokota S. Interleukin 6 as a therapeutic target in systemic-onset juvenile idiopathic arthritis. Curr Opin Rheumatol. 2003;15:581–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous