Arginase: a critical regulator of nitric oxide synthesis and vascular function - PubMed (original) (raw)
Review
Arginase: a critical regulator of nitric oxide synthesis and vascular function
William Durante et al. Clin Exp Pharmacol Physiol. 2007 Sep.
Abstract
1. Arginase is the focal enzyme of the urea cycle hydrolysing L-arginine to urea and L-ornithine. Emerging studies have identified arginase in the vasculature and have implicated this enzyme in the regulation of nitric oxide (NO) synthesis and the development of vascular disease. 2. Arginase inhibits the production of NO via several potential mechanisms, including competition with NO synthase (NOS) for the substrate L-arginine, uncoupling of NOS resulting in the generation of the NO scavenger, superoxide and peroxynitrite, repression of the translation and stability of inducible NOS protein, inhibition of inducible NOS activity via the generation of urea and by sensitization of NOS to its endogenous inhibitor asymmetric dimethyl-L-arginine. 3. Upregulation of arginase inhibits endothelial NOS-mediated NO synthesis and may contribute to endothelial dysfunction in hypertension, ageing, ischaemia-reperfusion and diabetes. 4. Arginase also redirects the metabolism of L-arginine to L-ornithine and the formation of polyamines and L-proline, which are essential for smooth muscle cell growth and collagen synthesis. Therefore, the induction of arginase may also promote aberrant vessel wall remodelling and neointima formation. 5. Arginase represents a promising novel therapeutic target that may reverse endothelial and smooth muscle cell dysfunction and prevent vascular disease.
Figures
Figure 1
Metabolism of L-arginine by nitric oxide synthase (NOS) and arginase. NOS oxidatively degrades L-arginine into L-citrulline and nitric oxide (NO) while arginase hydrolyzes L-arginine to urea and L-ornithine.
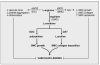
Figure 2
Model for the induction of vascular disease by arginase. In the circulation, the generation of NO by eNOS in endothelial cells (EC) plays a fundamental role in maintaining vascular homeostasis by inhibiting vascular tone, platelet aggregation, and inflammation. In addition, the iNOS-mediated formation of NO by vascular smooth muscle cells (SMC) functions in a negative feedback manner to inhibit collagen synthesis and SMC growth, and stimulate SMC apoptosis. However, arginase may promote both EC and SMC dysfunction by modulating the metabolism of L-arginine. In particular, the ability of arginase to compete with eNOS for the substrate L-arginine impairs NO synthesis and leads to endothelial dysfunction. In addition, arginase redirects the metabolism of L-arginine from NO to L-ornithine and the formation of polyamines and L-proline by the action of ornithine decarboxylase (ODC) and ornithine aminotransferase (OAT), respectively. The formation of polyamines and L-proline play an essential role in SMC proliferation and collagen deposition promoting the development of vascular lesions. These actions of arginase are further amplified by the repression of NO release, which serves as a well-established inhibitor of SMC growth and collagen synthesis.
Similar articles
- Arginase Inhibition Restores Peroxynitrite-Induced Endothelial Dysfunction via L-Arginine-Dependent Endothelial Nitric Oxide Synthase Phosphorylation.
Nguyen MC, Park JT, Jeon YG, Jeon BH, Hoe KL, Kim YM, Lim HK, Ryoo S. Nguyen MC, et al. Yonsei Med J. 2016 Nov;57(6):1329-38. doi: 10.3349/ymj.2016.57.6.1329. Yonsei Med J. 2016. PMID: 27593859 Free PMC article. - Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal?
Pernow J, Jung C. Pernow J, et al. Cardiovasc Res. 2013 Jun 1;98(3):334-43. doi: 10.1093/cvr/cvt036. Epub 2013 Feb 14. Cardiovasc Res. 2013. PMID: 23417041 Review. - In hepatocytes the regulation of NOS-2 activity at physiological L-arginine levels suggests a close link to the urea cycle.
Lerzynski G, Suschek CV, Kolb-Bachofen V. Lerzynski G, et al. Nitric Oxide. 2006 Jun;14(4):300-8. doi: 10.1016/j.niox.2005.11.009. Epub 2006 Jan 10. Nitric Oxide. 2006. PMID: 16410053 - Arginine attenuates methylglyoxal- and high glucose-induced endothelial dysfunction and oxidative stress by an endothelial nitric-oxide synthase-independent mechanism.
Dhar I, Dhar A, Wu L, Desai K. Dhar I, et al. J Pharmacol Exp Ther. 2012 Jul;342(1):196-204. doi: 10.1124/jpet.112.192112. Epub 2012 Apr 19. J Pharmacol Exp Ther. 2012. PMID: 22518022 - Arginase: A Multifaceted Enzyme Important in Health and Disease.
Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB. Caldwell RW, et al. Physiol Rev. 2018 Apr 1;98(2):641-665. doi: 10.1152/physrev.00037.2016. Physiol Rev. 2018. PMID: 29412048 Free PMC article. Review.
Cited by
- KCl-Dependent Release of Mitochondrial Membrane-Bound Arginase Appears to Be a Novel Variant of Arginase-II.
Suman M, Rajnikant M. Suman M, et al. Scientifica (Cairo). 2016;2016:3675283. doi: 10.1155/2016/3675283. Epub 2016 May 16. Scientifica (Cairo). 2016. PMID: 27293971 Free PMC article. - L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer's Disease.
Polis B, Srikanth KD, Elliott E, Gil-Henn H, Samson AO. Polis B, et al. Neurotherapeutics. 2018 Oct;15(4):1036-1054. doi: 10.1007/s13311-018-0669-5. Neurotherapeutics. 2018. PMID: 30288668 Free PMC article. - Relationship of Transforming Growth Factor-βl and Arginase-1 Levels with Long-term Survival after Kidney Transplantation.
Du XX, Guo YL, Yang M, Yu Y, Chang S, Liu B, Cai LJ, Chen ZK. Du XX, et al. Curr Med Sci. 2018 Jun;38(3):455-460. doi: 10.1007/s11596-018-1900-7. Epub 2018 Jun 22. Curr Med Sci. 2018. PMID: 30074212 - Arginine and Arginases Modulate Metabolism, Tumor Microenvironment and Prostate Cancer Progression.
Matos A, Carvalho M, Bicho M, Ribeiro R. Matos A, et al. Nutrients. 2021 Dec 16;13(12):4503. doi: 10.3390/nu13124503. Nutrients. 2021. PMID: 34960055 Free PMC article. Review. - Plasmatic Concentrations of ADMA and Homocystein in Llama (Lama glama) and Regulation of Arginase Type II: An Animal Resistent to the Development of Pulmonary Hypertension Induced by Hypoxia.
López V, Moraga FA, Llanos AJ, Ebensperger G, Taborda MI, Uribe E. López V, et al. Front Physiol. 2018 May 29;9:606. doi: 10.3389/fphys.2018.00606. eCollection 2018. Front Physiol. 2018. PMID: 29896110 Free PMC article.
References
- Barbul B. Arginine: biochemistry, physiology, and therapeutic implications. J Parenter Enteral Nutr. 1986;10:227–238. - PubMed
- Palmer RMJ, Ashton AS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1998;333:664–666. - PubMed
- Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;35:87–104. - PubMed
- Durante W. Regulation of L-arginine transport and metabolism in vascular smooth muscle cells. Cell Biochem Biophys. 2001;35:19–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR017659/RR/NCRR NIH HHS/United States
- R01 HL76187/HL/NHLBI NIH HHS/United States
- R01 HL074966/HL/NHLBI NIH HHS/United States
- R01 HL74966/HL/NHLBI NIH HHS/United States
- R01 HL076187/HL/NHLBI NIH HHS/United States
- R01 HL59976/HL/NHLBI NIH HHS/United States
- R01 HL074966-02/HL/NHLBI NIH HHS/United States
- R01 HL059976/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources