Reversibility in nucleocytoplasmic transport - PubMed (original) (raw)
Reversibility in nucleocytoplasmic transport
Ronen Benjamine Kopito et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Nucleocytoplasmic exchange of proteins and RNAs is mediated by receptors that usher their cargo through the nuclear pores. Peptide localization signals on each cargo determine the receptors with which it will interact. Those interactions are normally regulated by the small GTPase Ran. Hydrolysis of GTP provides the chemical energy required to create a bona fide thermodynamic pump that selectively and directionally accumulates its substrates across the nuclear envelope. A common perception is that cargo delivery is irreversible, e.g., a protein imported to the nucleus does not return to the cytoplasm except perhaps via a specific export receptor. Quantitative measurements using cell-free nuclei reconstituted in Xenopus egg extract show that nuclear accumulation follows first-order kinetics and reaches steady state at a level that follows a Michaelis-Menten function of the cytoplasmic cargo concentration. This saturation suggests that receptor-mediated translocation across the nuclear pore occurs bidirectionally. The reversibility of accumulation was demonstrated directly by exchange of the cytosolic medium and by fluorescence recovery after photobleaching. Based on our results, we offer a simple biophysical model that predicts the observed behavior. A far-reaching consequence is that the nuclear localization signal dictates the fate of a protein population rather than that of the individual molecules that bear it, which remain free to shuttle back and forth. This implies an open communication between the nucleus and cytoplasm and a ubiquitous mechanism for signaling in both directions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
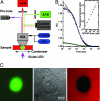
Experimental system. (A) Diagram of the multimode microscope, which incorporates transmission differential interference contrast imaging with simultaneous confocal epifluorescence intensity and FCS measurements, as well as wide-field epifluorescence imaging to a cooled CCD camera. A Zeiss ×40/N.A. 1.2 C-Apochromat water-immersion objective was used for all measurements. APD, avalanche photodiode; HID, metal-halide discharge lamp. See a detailed description in
SI Text
. (B) FCS was used to verify molecular mobility of the nuclear import substrate GFP-NP and to calibrate its local concentration in the nucleus (high concentration, 360 nM, in green) or cytosol (low concentration, 30 nM, in blue). Inflections at 1.00 and 0.96 msec, respectively, indicate similar diffusion constants in the two environments. Both fits are improved by an anomalous diffusion model, 〈_X_2〉 ∞ _t_γ, where γ = 0.85 and 0.75, respectively (data not shown). (Inset) Linearity of confocal fluorescence intensity with GFP-NP concentration in Xenopus egg extract. Filled circles represent quantitative calibration by FCS, and stars show concentration estimated by dilution. (C) Nuclear accumulation of GFP-NP (green, Left), differential interference contrast imaging (Center), and exclusion of tetramethyl rhodamine-conjugated dextran (red, Right) to verify integrity of the NE.
Fig. 2.
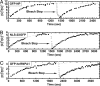
Nuclear accumulation kinetics. (A) Representative curves showing the time course of nuclear concentration for dilute cargo reservoirs (36 nM to 180 nM). Dotted lines are fits to simple first-order kinetics. (B) Representative curves showing the nuclear concentration, [_C_]N, normalized by the cytosolic concentration, [_C_]C. Up to 1.3 μM the kinetics were similar (shaded circles, 36 nM; others as marked). At higher concentrations both initial rates and steady-state ratios decreased. RanQ69L inhibited nuclear accumulation, which settled at a concentration ≈40% of the cytosolic concentration ([_C_]C = 400 nM).
Fig. 3.
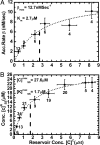
M-M behavior of GFP-NP accumulation. (A) The initial accumulation rates β are plotted here as a function of the cytosolic reservoir concentration, [_C_]C, after normalization to account for the nuclear volume. The dotted line shows the M-M curve fit: β = βmax([_C_]C/_K_m + [_C_]C), with βmax = 12.7 nM/sec and _K_m = 2.7 μM. (B) Steady-state nuclear cargo concentration is plotted as a function of the experimentally fixed cytoplasmic concentration, [_C_]C. Again the data follow a M-M form: [_C_]SSN = [_C_]_SS_max([_C_]C/([_K_]mSS + [_C_]C) with [_K_]mSS = 1.7 μM and [_C_]_SS_max = 27.6 μM. Vertical bars in both plots represent standard deviations from the number of measurements indicated. Horizontal bars show the concentration range over which the measurements were pooled for clarity.
Fig. 4.
Counterflow of GFP-NP on dilution. (A) Nuclei that had reached steady state in accumulation of GFP-NP ([_C_]C = 600 nM) were transferred to a new extract with [_C_]C reduced by a factor of 5. GFP-NP left the nucleus, and its concentration resettled at a new steady state. (See also
SI Movie 2
.) (B) The counterflow assay was performed for dilutions factors of 2 (circles), 2.5 (squares), and 5 (triangles), all beginning from 600 nM original cytosolic concentration of GFP-NP. In each case the nuclear concentration dropped by the same factor, so their ratios follow a line of slope unity (dashed line).
Fig. 5.
Photobleaching demonstrates counterflow kinetics noninvasively. Nuclear accumulation of transport substrates [GFP-NP (A), 1.3 μM; NLS-2×GFP (B), 1.5 μM; GFP-hnRNP A1 (C), 1.5 μM] approached saturation with first-order kinetics. After photobleaching, the reaccumulation of fresh fluorescent substrate followed the original uptake kinetics closely, as opposed to continuing the saturation curve that would be expected for irreversible, strictly vectorial import (dashed line in A). (See also
SI Movie 3
.)
Similar articles
- Nucleocytoplasmic shuttling of soluble tubulin in mammalian cells.
Akoumianaki T, Kardassis D, Polioudaki H, Georgatos SD, Theodoropoulos PA. Akoumianaki T, et al. J Cell Sci. 2009 Apr 15;122(Pt 8):1111-8. doi: 10.1242/jcs.043034. Epub 2009 Mar 19. J Cell Sci. 2009. PMID: 19299461 - Nuclear localization signals and human disease.
McLane LM, Corbett AH. McLane LM, et al. IUBMB Life. 2009 Jul;61(7):697-706. doi: 10.1002/iub.194. IUBMB Life. 2009. PMID: 19514019 Review. - Modulation of nucleocytoplasmic trafficking by retention in cytoplasm or nucleus.
Roth DM, Harper I, Pouton CW, Jans DA. Roth DM, et al. J Cell Biochem. 2009 Aug 15;107(6):1160-7. doi: 10.1002/jcb.22218. J Cell Biochem. 2009. PMID: 19507231 - Nucleocytoplasmic transport and nuclear envelope integrity in the fission yeast Schizosaccharomyces pombe.
Yoshida M, Sazer S. Yoshida M, et al. Methods. 2004 Jul;33(3):226-38. doi: 10.1016/j.ymeth.2003.11.018. Methods. 2004. PMID: 15157890 - Macromolecular exchanges between the nucleus and cytoplasm.
Feldherr CM. Feldherr CM. J Cell Biochem Suppl. 1998;30-31:214-9. J Cell Biochem Suppl. 1998. PMID: 9893273 Review.
Cited by
- Simple rules for passive diffusion through the nuclear pore complex.
Timney BL, Raveh B, Mironska R, Trivedi JM, Kim SJ, Russel D, Wente SR, Sali A, Rout MP. Timney BL, et al. J Cell Biol. 2016 Oct 10;215(1):57-76. doi: 10.1083/jcb.201601004. Epub 2016 Oct 3. J Cell Biol. 2016. PMID: 27697925 Free PMC article. - A discontinuous Galerkin model for fluorescence loss in photobleaching of intracellular polyglutamine protein aggregates.
Hansen CV, Schroll HJ, Wüstner D. Hansen CV, et al. BMC Biophys. 2018 Nov 29;11:7. doi: 10.1186/s13628-018-0046-0. eCollection 2018. BMC Biophys. 2018. PMID: 30519460 Free PMC article. - Viral subversion of the nuclear pore complex.
Le Sage V, Mouland AJ. Le Sage V, et al. Viruses. 2013 Aug 16;5(8):2019-42. doi: 10.3390/v5082019. Viruses. 2013. PMID: 23959328 Free PMC article. Review. - Thermodynamic Paradigm for Solution Demixing Inspired by Nuclear Transport in Living Cells.
Wang CH, Mehta P, Elbaum M. Wang CH, et al. Phys Rev Lett. 2017 Apr 14;118(15):158101. doi: 10.1103/PhysRevLett.118.158101. Epub 2017 Apr 10. Phys Rev Lett. 2017. PMID: 28452496 Free PMC article. - Dissecting in vivo steady-state dynamics of karyopherin-dependent nuclear transport.
Lolodi O, Yamazaki H, Otsuka S, Kumeta M, Yoshimura SH. Lolodi O, et al. Mol Biol Cell. 2016 Jan 1;27(1):167-76. doi: 10.1091/mbc.E15-08-0601. Epub 2015 Nov 4. Mol Biol Cell. 2016. PMID: 26538027 Free PMC article.
References
- Bagley S, Goldberg MW, Cronshaw JM, Rutherford S, Allen TD. J Cell Sci. 2000;113:3885–3886. - PubMed
- Rout MP, Aitchison JD. J Biol Chem. 2001;276:16593–16596. - PubMed
- Vasu SK, Forbes DJ. Curr Opin Cell Biol. 2001;13:363–375. - PubMed
- Pemberton LF, Paschal BM. Traffic. 2005;6:187–198. - PubMed
- Gorlich D, Kutay U. Annu Rev Cell Dev Biol. 1999;15:607–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous