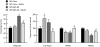Metabolic plasticity during mammalian development is directionally dependent on early nutritional status - PubMed (original) (raw)
Metabolic plasticity during mammalian development is directionally dependent on early nutritional status
Peter D Gluckman et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Developmental plasticity in response to environmental cues can take the form of polyphenism, as for the discrete morphs of some insects, or of an apparently continuous spectrum of phenotype, as for most mammalian traits. The metabolic phenotype of adult rats, including the propensity to obesity, hyperinsulinemia, and hyperphagia, shows plasticity in response to prenatal nutrition and to neonatal administration of the adipokine leptin. Here, we report that the effects of neonatal leptin on hepatic gene expression and epigenetic status in adulthood are directionally dependent on the animal's nutritional status in utero. These results demonstrate that, during mammalian development, the direction of the response to one cue can be determined by previous exposure to another, suggesting the potential for a discontinuous distribution of environmentally induced phenotypes, analogous to the phenomenon of polyphenism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
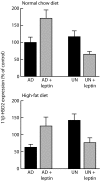
Fig. 1.
Fetal nutrition induces a bifurcation in the response of 11β-HSD2 gene expression to leptin treatment. We measured mRNA expression of the 11β-HSD2 gene in the livers of adult (170-day-old) female rats exposed in utero to maternal undernutrition (UN) or ad libitum feeding (AD), treated with saline or leptin from days 3–10 of life, and then fed normal chow (Upper) or a high-fat diet (Lower) from weaning. Maternal nutrition altered the direction of the response of 11β-HSD2 expression to neonatal leptin treatment (test for reversal of sign of response, P < 0.01; test for interaction between prenatal nutrition and leptin administration, P < 0.0001); the effect was independent of postweaning nutrition. Data are means ± SEM for n = 8 per group; values are expressed relative to that of normally nourished (maternally ad libitum-fed and postweaning chow-fed) and saline-treated offspring set as 100%.
Fig. 2.
Neonatal leptin treatment normalizes gross phenotype (adiposity) and hepatic gene expression in adult rats exposed to undernutrition in utero followed by hypercaloric nutrition after weaning. We measured total body fat (dual-energy x-ray absorptiometry) (Left) and hepatic mRNA expression (Right) of adult (170-day-old) female rats that had been subject in utero to maternal undernutrition (UN) or ad libitum feeding (AD), treated with saline or leptin from days 3–10 of life, and then fed normal chow or a high-fat diet from weaning. Data are means ± SEM for n = 8 per group; values for gene expression are expressed relative to those of normally nourished (maternally ad libitum-fed and postweaning chow-fed) and saline-treated offspring set as 100%. Data for total body fat are replotted from Vickers et al. (13). *, P < 0.05: significantly different from normally nourished, saline-treated animals. †, P < 0.05: significant effect of leptin treatment in UN high-fat animals.
Similar articles
- The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy.
Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Vickers MH, et al. Endocrinology. 2008 Apr;149(4):1906-13. doi: 10.1210/en.2007-0981. Epub 2008 Jan 10. Endocrinology. 2008. PMID: 18187552 - Neonatal leptin treatment reverses developmental programming.
Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Vickers MH, et al. Endocrinology. 2005 Oct;146(10):4211-6. doi: 10.1210/en.2005-0581. Epub 2005 Jul 14. Endocrinology. 2005. PMID: 16020474 - Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments.
Guilloteau P, Zabielski R, Hammon HM, Metges CC. Guilloteau P, et al. J Physiol Pharmacol. 2009 Oct;60 Suppl 3:17-35. J Physiol Pharmacol. 2009. PMID: 19996479 Review. - The effect of nutrition during early life on the epigenetic regulation of transcription and implications for human diseases.
Lillycrop KA, Burdge GC. Lillycrop KA, et al. J Nutrigenet Nutrigenomics. 2011;4(5):248-60. doi: 10.1159/000334857. Epub 2012 Feb 22. J Nutrigenet Nutrigenomics. 2011. PMID: 22353662 Review.
Cited by
- Recent developments on the role of epigenetics in obesity and metabolic disease.
van Dijk SJ, Tellam RL, Morrison JL, Muhlhausler BS, Molloy PL. van Dijk SJ, et al. Clin Epigenetics. 2015 Jul 11;7:66. doi: 10.1186/s13148-015-0101-5. eCollection 2015. Clin Epigenetics. 2015. PMID: 27408648 Free PMC article. Review. - Post-weaning selenium and folate supplementation affects gene and protein expression and global DNA methylation in mice fed high-fat diets.
Bermingham EN, Bassett SA, Young W, Roy NC, McNabb WC, Cooney JM, Brewster DT, Laing WA, Barnett MP. Bermingham EN, et al. BMC Med Genomics. 2013 Mar 5;6:7. doi: 10.1186/1755-8794-6-7. BMC Med Genomics. 2013. PMID: 23497688 Free PMC article. - Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction.
Theys N, Clippe A, Bouckenooghe T, Reusens B, Remacle C. Theys N, et al. PLoS One. 2009 Jul 1;4(7):e6110. doi: 10.1371/journal.pone.0006110. PLoS One. 2009. PMID: 19568427 Free PMC article. - Diet, nutrition and modulation of genomic expression in fetal origins of adult disease.
Jackson AA, Burdge GC, Lillicrop KA. Jackson AA, et al. J Nutrigenet Nutrigenomics. 2010;3(4-6):192-208. doi: 10.1159/000324356. Epub 2011 Apr 6. J Nutrigenet Nutrigenomics. 2010. PMID: 21474951 Free PMC article. No abstract available. - Complex genetics of obesity in mouse models.
Pomp D, Nehrenberg D, Estrada-Smith D. Pomp D, et al. Annu Rev Nutr. 2008;28:331-45. doi: 10.1146/annurev.nutr.27.061406.093552. Annu Rev Nutr. 2008. PMID: 18435591 Free PMC article. Review.
References
- West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003.
- Applebaum SW, Heifetz Y. Annu Rev Entomol. 1999;44:317–341. - PubMed
- Gluckman PD, Hanson MA, Beedle AS. Am J Hum Biol. 2007;19:1–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources