Control of excitatory synaptic transmission by capsaicin is unaltered in TRPV1 vanilloid receptor knockout mice - PubMed (original) (raw)
Control of excitatory synaptic transmission by capsaicin is unaltered in TRPV1 vanilloid receptor knockout mice
Felix Benninger et al. Neurochem Int. 2008 Jan.
Abstract
Several studies have shown that capsaicin could effectively regulate excitatory synaptic transmission in the central nervous system, but the assumption that this effect is mediated by TRPV1 vanilloid receptors (TRPV1Rs) has not been tested directly. To provide direct evidence, we compared the effect of capsaicin on excitatory synapses in wild type mice and TRPV1R knockouts. Using whole-cell patch-clamp techniques, excitatory postsynaptic currents (EPSCs) were recorded in granule cells of the dentate gyrus. First, we investigated the effect of capsaicin on EPSCs evoked by focal stimulation of fibers in the stratum moleculare. Bath application of 10 microM capsaicin reduced the amplitude of evoked EPSCs both in wild type and TRPV1R knockout animals to a similar extent. Treatment of the slices with the TRPV1R antagonist capsazepine (10 microM) alone, or together with the agonist capsaicin, also caused a decrease in the EPSC amplitude both in wild type and TRPV1R knockout animals. Both drugs appeared to affect the efficacy of excitatory synapses at presynaptic sites, since a significant increase was observed in paired-pulse ratio of EPSC amplitude after drug treatment. Next we examined the effect of capsaicin on spontaneously occurring EPSCs. This prototypic vanilloid ligand increased the frequency of events without changing their amplitude in wild type mice. Similar enhancement in the frequency without altering the amplitude of spontaneous EPSCs was observed in TRPV1R knockout mice. These data strongly argue against the hypothesis that capsaicin modulates excitatory synaptic transmission by activating TRPV1Rs, at least in the hippocampal network.
Figures
Fig. 1
Results of genotyping a litter. Presence of a 150 bp length fragment indicates wild type allele, 300 bp PCR fragment shows targeted allele. TRPV1Rs (+/+, wild type;+/−, heterozygote; −/−, knockout). Controls (ctr) for +/+ and −/− are also indicated.
Fig. 2
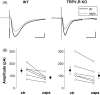
The suppression of excitatory postsynaptic currents by capsaicin in dentate granule cells recorded in wild type (WT) or TRPV1R knockout (KO) mice. (A): Representative averaged recordings of six to eight consecutive EPSCs taken before (black) and after application of 10 μM capsaicin (gray) in wild type mice (WT) or in TRPV1R knockouts. Scale bars are 25 pA and 10 ms. (B): Individual values (open circles) and averaged data (solid circles) for the capsaicin-induced suppression of EPSCs in wild type and TRPV1R knockout mice are shown.
Fig. 3
The reduction of excitatory postsynaptic currents by capsazepine (CZ) in dentate granule cells recorded in wild type (WT) or TRPV1R knockout (KO) mice. (A): Representative averaged recordings of 8–10 consecutive EPSCs taken before (black) and after application of 10 μM capsazepine (gray) in wild type mice (WT) or in TRPV1R knockouts. Scale bars are 25 pA and 10 ms. (B): Individual values (open circles) and averaged data (solid circles) for the capsazepine-induced suppression of EPSCs in wild type and TRPV1R knockout mice are shown.
Fig. 4
The occurrence of spontaneous EPSCs in dentate granule cells is increased by capsaicin both in wild type and TRPV1R knockout mice. (A): Representative raw recordings before and after bath application of 10 μM capsaicin. Scale bars are 10 pA and 100 ms. (B): Median of individual experiments (open circles) and averaged values (filled circles) for the inter-event interval (IEI) and the amplitude of spontaneous EPSCs in control and after drug application are shown. Capsaicin induced a significant reduction in the inter-event intervals of synaptic events (i.e., increased the frequency) without changing their amplitude both in wild type and TRPV1R knockout mice.
Similar articles
- Expression of TRPV1 channels by Cajal-Retzius cells and layer-specific modulation of synaptic transmission by capsaicin in the mouse hippocampus.
Anstötz M, Lee SK, Maccaferri G. Anstötz M, et al. J Physiol. 2018 Aug;596(16):3739-3758. doi: 10.1113/JP275685. Epub 2018 Jun 24. J Physiol. 2018. PMID: 29806907 Free PMC article. - Presynaptic TRPV1 vanilloid receptor function is age- but not CB1 cannabinoid receptor-dependent in the rodent forebrain.
Köles L, Garção P, Zádori ZS, Ferreira SG, Pinheiro BS, da Silva-Santos CS, Ledent C, Köfalvi A. Köles L, et al. Brain Res Bull. 2013 Aug;97:126-35. doi: 10.1016/j.brainresbull.2013.06.007. Epub 2013 Jul 4. Brain Res Bull. 2013. PMID: 23831917 - Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers.
Hájos N, Freund TF. Hájos N, et al. Neuropharmacology. 2002 Sep;43(4):503-10. doi: 10.1016/s0028-3908(02)00157-0. Neuropharmacology. 2002. PMID: 12367597 - Effect of resiniferatoxin on glutamatergic spontaneous excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord.
Jiang CY, Fujita T, Yue HY, Piao LH, Liu T, Nakatsuka T, Kumamoto E. Jiang CY, et al. Neuroscience. 2009 Dec 29;164(4):1833-44. doi: 10.1016/j.neuroscience.2009.09.033. Epub 2009 Sep 22. Neuroscience. 2009. PMID: 19778582 - Enhanced ability of TRPV1 channels in regulating glutamatergic transmission after repeated morphine exposure in the nucleus accumbens of rat.
Zhang H, Jia D, Wang Y, Qu L, Wang X, Song J, Heng L, Gao G. Zhang H, et al. Brain Res. 2017 Apr 1;1660:47-57. doi: 10.1016/j.brainres.2017.02.002. Epub 2017 Feb 7. Brain Res. 2017. PMID: 28188777
Cited by
- Activation of transient receptor potential vanilloid 1 ameliorates tau accumulation-induced synaptic damage and cognitive dysfunction via autophagy enhancement.
Zhang T, Tian Y, Zheng X, Li R, Hu L, Shui X, Mei Y, Wang Q, Zhang M, Zheng X, Wang L, Chen D, Tao W, Lee TH. Zhang T, et al. CNS Neurosci Ther. 2024 Mar;30(3):e14432. doi: 10.1111/cns.14432. Epub 2023 Aug 29. CNS Neurosci Ther. 2024. PMID: 37641913 Free PMC article. - Expression of TRPV1 channels by Cajal-Retzius cells and layer-specific modulation of synaptic transmission by capsaicin in the mouse hippocampus.
Anstötz M, Lee SK, Maccaferri G. Anstötz M, et al. J Physiol. 2018 Aug;596(16):3739-3758. doi: 10.1113/JP275685. Epub 2018 Jun 24. J Physiol. 2018. PMID: 29806907 Free PMC article. - Endocannabinoid-mediated synaptic plasticity and addiction-related behavior.
Sidhpura N, Parsons LH. Sidhpura N, et al. Neuropharmacology. 2011 Dec;61(7):1070-87. doi: 10.1016/j.neuropharm.2011.05.034. Epub 2011 Jun 12. Neuropharmacology. 2011. PMID: 21669214 Free PMC article. Review. - Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways.
Starowicz K, Maione S, Cristino L, Palazzo E, Marabese I, Rossi F, de Novellis V, Di Marzo V. Starowicz K, et al. J Neurosci. 2007 Dec 12;27(50):13739-49. doi: 10.1523/JNEUROSCI.3258-07.2007. J Neurosci. 2007. PMID: 18077685 Free PMC article. - Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells.
Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Cavanaugh DJ, et al. J Neurosci. 2011 Mar 30;31(13):5067-77. doi: 10.1523/JNEUROSCI.6451-10.2011. J Neurosci. 2011. PMID: 21451044 Free PMC article.
References
- Acs G., Palkovits M., Blumberg P.M. Specific binding of [3H]resiniferatoxin by human and rat preoptic area, locus ceruleus, medial hypothalamus, reticular formation and ventral thalamus membrane preparations. Life Sci. 1996;59:1899–1908. - PubMed
- Al-Hayani A., Wease K.N., Ross R.A., Pertwee R.G., Davies S.N. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41:1000–1005. - PubMed
- Balla Z., Szoke E., Czeh G., Szolcsanyi J. Effect of capsaicin on voltage-gated currents of trigeminal neurones in cell culture and slice preparations. Acta Physiol. Hung. 2001;88:173–196. - PubMed
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
- Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources