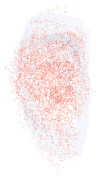Myofiber length and three-dimensional localization of NMJs in normal and botulinum toxin treated adult extraocular muscles - PubMed (original) (raw)
Comparative Study
Myofiber length and three-dimensional localization of NMJs in normal and botulinum toxin treated adult extraocular muscles
Andrew R Harrison et al. Invest Ophthalmol Vis Sci. 2007 Aug.
Abstract
Purpose: The density and three-dimensional localization of neuromuscular junctions (NMJs) of normal and botulinum toxin-treated normal adult rabbit and monkey extraocular muscles (EOMs) were analyzed. To demonstrate average myofiber length, randomly selected individual myofibers were reconstructed and compared with total muscle length.
Methods: Normal adult rabbit and monkey EOM and normal adult rabbit tibialis anterior were dissected in their entirety, frozen, sectioned longitudinally, and immunostained for NMJ localization. In addition, adult rabbit EOMs were injected with 5 U botulinum toxin, and NMJ density was determined after 2 weeks. NMJ locations for the three groups of EOM were reconstructed, and density of NMJ was determined. Individual myofibers were reconstructed from the orbital and global layers to determine mean fiber length.
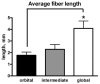
Results: NMJs were dispersed throughout the entire length of all EOMs examined from adult rabbits and monkeys and were visualized by alpha-bungarotoxin staining and three-dimensional reconstruction of serial sections. In leg muscle, two relatively tight bands of NMJs were seen. Botulinum toxin significantly increased total NMJ density. Mean fiber lengths were 1.9 and 4.83 mm in the orbital and global layers, respectively, approximately 10% and 24% of the total origin-to-insertion muscle lengths. In addition, individual myofibers continuously changed their intrafascicular relationships over their lengths.
Conclusions: The density and distribution of NMJs in normal EOMs are more extensive than previously described. Individual myofibers are significantly shorter than the tendon-to-tendon muscle length in both muscle layers. Botulinum toxin results in a doubling of NMJ density. NMJ localization in normal EOMs has ramifications for understanding eye movement control, but it is also important when surgical or pharmacologic intervention is used for the treatment of strabismus, nystagmus, or other eye muscle disorders.
Figures
Figure 1
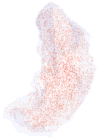
(A) Single longitudinal section taken from the middle of the muscle thickness, and thus the global layer, of a control superior rectus muscle from a normal adult rabbit. All the NMJs marked in red are oriented with the origin and insertion at the top and bottom of the reconstruction. (B) Single longitudinal section taken from the middle of the thickness, and thus the global layer, of a superior rectus muscle from an adult rabbit 2 weeks after a single injection of 5 U botulinum toxin. All the NMJs marked in red are oriented with the origin and insertion at the top and bottom of the reconstruction.
Figure 2
Three-dimensional reconstruction of all the _α_-bungarotoxin–positive NMJs in every 10th section through the entire thickness of a single control superior rectus muscle from an adult rabbit. All NMJs are indicated in red, and the specimen is oriented with the origin and insertion at the top and bottom of the reconstruction.
Figure 3
Three-dimensional reconstruction of all the _α_-bungarotoxin–positive NMJs in every 10th section through the entire thickness of a single control superior rectus muscle from an adult monkey. All the NMJs are indicated in red, and the specimen is oriented with the origin and insertion at the top and bottom of the reconstruction.
Figure 4
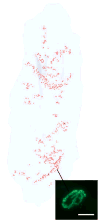
Three-dimensional reconstruction of all the _α_-bungarotoxin–positive NMJs in every 20th section through the entire thickness of a single control tibialis anterior muscle from an adult rabbit. All the NMJs are indicated in red, and the specimen is oriented with the origin and insertion at the top and bottom of the reconstruction. Inset is an example of an _α_-bungarotoxin–labeled NMJ. Total length of rabbit tibialis muscle is approximately 5.5 cm.
Figure 5
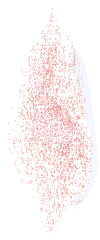
Three-dimensional reconstruction of all the _α_-bungarotoxin–positive NMJs in every 10th section through the entire thickness of a single superior rectus muscle from a rabbit 2 weeks after a single injection of 5 U botulinum toxin. All the NMJs are indicated in red, and the specimen is oriented with the origin and insertion at the top and bottom of the reconstruction.
Figure 6
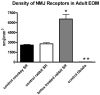
Graph of the mean NMJ density in cubic millimeters in control monkey (black), control rabbit (white), and botulinum toxin–treated (gray) superior rectus (SR) muscles. *Significant difference from control at P < 0.005. **Density is low (18.9 ± 0.54/mm3) and thus is not clearly visible when graphed compared with NMJ density within the normal EOM of rabbit and monkey.
Figure 7
Representative examples of individual myofibers reconstructed from the (A) orbital layer, (B) global layer directly contiguous with the orbital layer, and (C) middle of the global layer. These represent the complete lengths of these three fibers followed in serial sections.
Figure 8
Mean myofiber length, in millimeters, of individual myofibers reconstructed from the orbital layer (black), global layer directly contiguous with the orbital layer (gray), and middle of the global layer (white). *Significant difference from orbital and intermediate layer fibers compared with fibers from the middle of the global layer (P < 0.005).
Figure 9
(A–O) Representative photomicrographs of a single fiber from an adult normal superior rectus muscle (4.622 mm long) from rabbit, demonstrating changes in its fascicular relationship with its neighboring fibers relatively often in the course of its fiber length. *Individual myofiber that was reconstructed. Numbered fibers allow observation of the changes in the neighboring muscle fibers over the course of 2.9 mm represented by these selected sections. Horizontal arrows indicate fibers that newly appear in the cross-sections within 2.9 mm of the reconstruction (numbers 9 and 10). Vertical arrow indicates a fiber that ends within 2.9 mm.
Similar articles
- Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling.
Ugalde I, Christiansen SP, McLoon LK. Ugalde I, et al. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4114-20. doi: 10.1167/iovs.05-0549. Invest Ophthalmol Vis Sci. 2005. PMID: 16249488 Free PMC article. - Neuromuscular Junction Development Differs Between Extraocular and Skeletal Muscles and Between Different Extraocular Muscles.
Vemula S, Muvavarirwa T, Doornbos F, Whitman MC. Vemula S, et al. Invest Ophthalmol Vis Sci. 2024 May 1;65(5):28. doi: 10.1167/iovs.65.5.28. Invest Ophthalmol Vis Sci. 2024. PMID: 38767908 Free PMC article. - Impaired Extraocular Muscle Innervation Is Present Before Eye Opening in a Mouse Model of Infantile Nystagmus Syndrome.
Vemula SK, Kim SA, Muvavarirwa T, Bell JL, Whitman MC. Vemula SK, et al. Invest Ophthalmol Vis Sci. 2022 Sep 1;63(10):4. doi: 10.1167/iovs.63.10.4. Invest Ophthalmol Vis Sci. 2022. PMID: 36083589 Free PMC article. - Current clinical applications of botulinum toxin.
Truong DD, Stenner A, Reichel G. Truong DD, et al. Curr Pharm Des. 2009;15(31):3671-80. doi: 10.2174/138161209789271843. Curr Pharm Des. 2009. PMID: 19925419 Review. - Botulinum toxin type B: an overview of its biochemistry and preclinical pharmacology.
Callaway JE, Arezzo JC, Grethlein AJ. Callaway JE, et al. Dis Mon. 2002 May;48(5):367-83. doi: 10.1053/mda.2001.24421. Dis Mon. 2002. PMID: 12195266 Review.
Cited by
- Morphological Differences in the Inferior Oblique Muscles from Subjects with Over-elevation in Adduction.
Rudell JC, Stager D Jr, Felius J, McLoon LK. Rudell JC, et al. Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):33. doi: 10.1167/iovs.61.6.33. Invest Ophthalmol Vis Sci. 2020. PMID: 32539136 Free PMC article. - Strabismus and the Oculomotor System: Insights from Macaque Models.
Das VE. Das VE. Annu Rev Vis Sci. 2016 Oct 14;2:37-59. doi: 10.1146/annurev-vision-111815-114335. Epub 2016 Jul 18. Annu Rev Vis Sci. 2016. PMID: 28532347 Free PMC article. - Botulinum toxin pretreatment augments the weakening effect of injection with ricin-mAb35 in rabbit extraocular muscle.
Christiansen SP, Anderson BC, McLoon LK. Christiansen SP, et al. J AAPOS. 2008 Apr;12(2):122-7. doi: 10.1016/j.jaapos.2007.11.001. Epub 2008 Feb 7. J AAPOS. 2008. PMID: 18258470 Free PMC article. - Dynamic Electrooculography Findings for Medial Rectus Myofascial Release in Esodeviation.
Mohamadi A, Vasaghi-Gharamaleki B, Mirzajani A, Jafarzadehpur E. Mohamadi A, et al. J Curr Ophthalmol. 2023 Dec 21;35(2):190-194. doi: 10.4103/joco.joco_143_23. eCollection 2023 Apr-Jun. J Curr Ophthalmol. 2023. PMID: 38250489 Free PMC article. - Differential Vulnerability of Oculomotor Versus Hypoglossal Nucleus During ALS: Involvement of PACAP.
Maugeri G, D'Amico AG, Morello G, Reglodi D, Cavallaro S, D'Agata V. Maugeri G, et al. Front Neurosci. 2020 Aug 11;14:805. doi: 10.3389/fnins.2020.00805. eCollection 2020. Front Neurosci. 2020. PMID: 32848572 Free PMC article. Review.
References
- Happak W, Liu J, Burggasser G, Flowers A, Gruber H, Freilinger G. Human facial muscles: dimensions, motor endplate distribution and presence of muscle fibers with multiple motor endplates. Anat Rec. 1997;249:276–284. - PubMed
- Lander T, Wirtschafter JD, McLoon LK. Orbicularis oculi muscle fibers are relatively short and heterogeneous in length. Invest Ophthalmol Vis Sci. 1996;37:1732–1739. - PubMed
- Rossi G, Cortesina G. Morphological study of the laryngeal muscles in man. Acta Otolaryngol. 1965;59:575–592. - PubMed
- Namba T, Nakamura T, Grob D. Motor nerve endings in human extraocular muscle. Neurology. 1968;18:403–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017768/AG/NIA NIH HHS/United States
- R03 EY013979/EY/NEI NIH HHS/United States
- R01 EY015313/EY/NEI NIH HHS/United States
- EY13979/EY/NEI NIH HHS/United States
- EY11375/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources