Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration - PubMed (original) (raw)
. 2007 Aug;48(8):3827-36.
doi: 10.1167/iovs.07-0051.
Christine M Bojanowski, Min Zhou, Defen Shen, Robert J Ross, Kevin I Rosenberg, D Joshua Cameron, Chunyue Yin, Jeffrey A Kowalak, Zhengping Zhuang, Kang Zhang, Chi-Chao Chan
Affiliations
- PMID: 17652758
- PMCID: PMC2048751
- DOI: 10.1167/iovs.07-0051
Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration
Jingsheng Tuo et al. Invest Ophthalmol Vis Sci. 2007 Aug.
Abstract
Purpose: Senescent Ccl2(-/-) mice are reported to develop cardinal features of human age-related macular degeneration (AMD). Loss-of-function single-nucleotide polymorphisms within CX3CR1 are also found to be associated with AMD. The authors generated Ccl2(-/-)/Cx3cr1(-/-) mice to establish a more characteristic and reproducible AMD model.
Methods: Single Ccl2- and Cx3cr1-deficient mice were crossbred to obtain Ccl2(-/-)/Cx3cr1(-/-) mice. Funduscopy, histopathology, retinal A2E quantification, proteomics, RT-PCR gene expression assay, immunochemistry, and Western blotting were used to examine the retina and to evaluate gene expression within the retinal tissue.
Results: By 6 weeks of age, all Ccl2(-/-)/Cx3cr1(-/-) mice developed AMD-like retinal lesions, including drusen, retinal pigment epithelium alteration, and photoreceptor degeneration. Furthermore, choroidal neovascularization occurred in 15% of the mice. These degenerative lesions progressed with age. A2E, a major lipofuscin fluorophore that accumulated during AMD progression, was significantly higher in the Ccl2(-/-)/Cx3cr1(-/-) retina than in the wild-type retina. Complement cofactor was higher in the Ccl2(-/-)/Cx3cr1(-/-) RPE. Proteomics data indicated that four proteins were differentially expressed in Ccl2(-/-)/Cx3cr1(-/-) retina compared with control. One of these proteins, ERp29, an endoplasmic reticulum protein, functions as an escort chaperone and in protein folding.
Conclusions: The authors concluded that Ccl2(-/-)/Cx3cr1(-/-) mice develop a broad spectrum of AMD abnormalities with early onset and high penetrance. These observations implicate certain chemokines and endoplasmic reticulum proteins in AMD pathogenesis. Similar to the mechanism of neurodegeneration caused by dysfunction of endoplasmic reticulum proteins, decreased chaperoning may cause misfolded protein accumulation, leading to drusen formation and retinal degeneration.
Figures
Figure 1
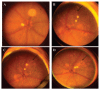
Fundus photographs. (A) A normal retina is illustrated in a 21-week-old WT mouse. (B) Multiple subretinal lesions mimic drusen formation (arrow) in a 9-week-old _Ccl2_-/-/_Cx3cr1_-/- mouse. (C) Enlarged and confluent subretinal drusenlike lesions (arrow) in the same eye of the _Ccl2_-/-/_Cx3cr1_-/- mouse at 18 weeks. (D) Scarring and atrophic retinal lesions (arrow) indicate disease progression in the same eye of this mouse at 33 weeks.
Figure 2
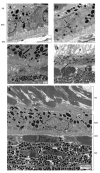
Ocular photomicrographs. (A) Retina, RPE, Bruch membrane, and choroidal capillaries are normal in a 6-month-old WT mouse. (B) Drusen deposits (arrows) are shown as small dome-shaped hyaline material within the Bruch membrane of two _Ccl2_-/-/_Cx3cr1_-/- mice. (C) Hypopigmentation and lacy degeneration (arrow) of the RPE are observed in a _Ccl2_-/-/_Cx3cr1_-/- mouse. (D) Choroidal neovascularization is identified by small patent vessels (arrows) from the choroid piercing Bruch membrane and RPE into the retina of two _Ccl2_-/-/_Cx3cr1_-/- mice. GCL, ganglion cell layer; INL, inner nuclear layer; PR, photoreceptor outer and inner segments; CH, choroids. Hematoxylin and eosin staining. Scale bar, 100 μm.
Figure 3
Retinal transmission electron micrographs. (A) Melanosomes are markedly decreased and lipofuscin is present in the RPE cells (arrow) of a _Ccl2_-/-/_Cx3cr1_-/- mouse. (B) Lipofuscin (arrow) and lipid droplets (open arrow) deposits are shown as the homogeneous electron dense-cytoplasmic inclusion in the RPE of another _Ccl2_-/-/_Cx3cr1_-/-. (C) Thickened Bruch membrane and amorphous deposits (arrow) are depicted in a _Ccl2_-/-/_Cx3cr1_-/- mouse. (D) Disorganized and atrophic photoreceptors (asterisk) and lipofuscin (arrow) in the RPE are observed. (E) Normal RPE, Bruch membrane, and choroid are illustrated in a WT mouse. Scale bars, 500 nm (A, B, D); 2 μm (C, E).
Figure 4
Quantification of A2E in _Ccl2_-/-/_Cx3cr1_-/- mice compared with normal controls. Chromatograms represent absorbance at 440 nm. A2E peaks elute at 25.5 minutes. _Ccl2_-/-/_Cx3cr1_-/- RPE at 4 months: A2E approximately 3.4 pmol (black). Normal RPE at 4 months: A2E approximately 1.1 pmol (gray). Each represents a 200-μL injection of six RPE extracts.
Figure 5
Ocular photomicrographs of complement cofactor and microglia. (A, C) CD46 (arrows, black) staining in entire RPE and small drusen of a _Ccl2_-/-/_Cx3cr1_-/- mouse compared with none in the retina of a WT mouse (open arrow, RPE with brown pigment). (B, D) Microglia (CD11b+ cells, arrows) are found in the retinal lesions of a _Ccl2_-/-/_Cx3cr1_-/- mouse; none are found in a WT mouse (open arrow, RPE). Insets: higher magnifications of the RPE cells in two strains. (A, B: WT mice; C, D: _Ccl2_-/-/_Cx3cr1_-/- mice). INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 100 μm.
Figure 6
Proteomics of retinal lysate from WT and _Ccl2_-/-/_Cx3cr1_-/- mice by two-dimensional PAGE analysis. (A) Representative two-dimensional PAGE image from one WT retina. (B) Representative two-dimensional PAGE image from one _Ccl2_-/-/_Cx3cr1_-/- retina. Four silver-stained protein spots are identified as differentially expressed between the two groups, as indicated by circles. The three proteins from those four spots were successfully identified by LC/MS/MS. Repetition of the analysis reveals consistent protein patterns for each group. The three identified proteins were (1) calcium binding 140k protein, (2) ERp29 precursor, and (3) RIKEN cDNA 2210010C04.
Figure 7
ERp29 expression in the retina. (A) Photomicrographs of mice retinal sections show less ERp29 reactivity (black, arrows) and presence of retinal lesions (asterisks)in a _Ccl2_-/-/_Cx3cr1_-/- mouse than in a WT mouse (avidin-biotin-immunoperoxidase staining). Scale bar, 100 μm. (B) Western blotting of retina lysate shows less density of ERp29 in a _Ccl2_-/-/_Cx3cr1_-/- mouse than in a WT mouse. (C) Lower ocular ERp29 mRNA detected by quantitative RT-PCR in the ocular tissue of _Ccl2_-/-/_Cx3cr1_-/- mice than in WT mice.
Figure 8
Photomicrographs of ERp29 in human eyes. (A) Positive ERp29 reactivity (arrows, black) is detected in the inner segments of photoreceptor layer, the junction of the outer plexiform and inner nuclear layers, the ganglion cell layer, and the RPE cells of a normal retina. (B) Immunoreactivity (arrows) was lower in an AMD eye, particularly in the diseased retinal outer layers and RPE cells, than in the normal eye. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; Pho, inner and outer segments of photoreceptor cells; C, choroids; S, sclera. Avidin-biotin-immunoperoxidase staining. Scale bar, 100 μm.
Similar articles
- Constancy of ERp29 expression in cultured retinal pigment epithelial cells in the Ccl2/Cx3cr1 deficient mouse model of age-related macular degeneration.
Verma V, Sauer T, Chan CC, Zhou M, Zhang C, Maminishkis A, Shen D, Tuo J. Verma V, et al. Curr Eye Res. 2008 Aug;33(8):701-7. doi: 10.1080/02713680802236185. Curr Eye Res. 2008. PMID: 18696346 Free PMC article. - Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration.
Chan CC, Ross RJ, Shen D, Ding X, Majumdar Z, Bojanowski CM, Zhou M, Salem N Jr, Bonner R, Tuo J. Chan CC, et al. Ophthalmic Res. 2008;40(3-4):124-8. doi: 10.1159/000119862. Epub 2008 Apr 18. Ophthalmic Res. 2008. PMID: 18421225 Free PMC article. - Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration.
Ross RJ, Zhou M, Shen D, Fariss RN, Ding X, Bojanowski CM, Tuo J, Chan CC. Ross RJ, et al. Exp Eye Res. 2008 Apr;86(4):675-83. doi: 10.1016/j.exer.2008.01.014. Epub 2008 Jan 25. Exp Eye Res. 2008. PMID: 18308304 Free PMC article. - Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD).
Ramkumar HL, Zhang J, Chan CC. Ramkumar HL, et al. Prog Retin Eye Res. 2010 May;29(3):169-90. doi: 10.1016/j.preteyeres.2010.02.002. Epub 2010 Mar 3. Prog Retin Eye Res. 2010. PMID: 20206286 Free PMC article. Review. - CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration.
Raoul W, Auvynet C, Camelo S, Guillonneau X, Feumi C, Combadière C, Sennlaub F. Raoul W, et al. J Neuroinflammation. 2010 Dec 2;7:87. doi: 10.1186/1742-2094-7-87. J Neuroinflammation. 2010. PMID: 21126357 Free PMC article. Review.
Cited by
- Progress and perspectives on the role of RPE cell inflammatory responses in the development of age-related macular degeneration.
Qin S, Rodrigues GA. Qin S, et al. J Inflamm Res. 2008;1:49-65. doi: 10.2147/jir.s4354. Epub 2008 Dec 2. J Inflamm Res. 2008. PMID: 22096347 Free PMC article. - Endoplasmic reticulum protein 29 protects cortical neurons from apoptosis and promoting corticospinal tract regeneration to improve neural behavior via caspase and Erk signal in rats with spinal cord transection.
Liu R, Zhao W, Zhao Q, Liu SJ, Liu J, He M, Xu Y, Wang W, Liu W, Xia QJ, Li CY, Wang TH. Liu R, et al. Mol Neurobiol. 2014 Dec;50(3):1035-48. doi: 10.1007/s12035-014-8681-1. Epub 2014 May 3. Mol Neurobiol. 2014. PMID: 24794144 - Constancy of ERp29 expression in cultured retinal pigment epithelial cells in the Ccl2/Cx3cr1 deficient mouse model of age-related macular degeneration.
Verma V, Sauer T, Chan CC, Zhou M, Zhang C, Maminishkis A, Shen D, Tuo J. Verma V, et al. Curr Eye Res. 2008 Aug;33(8):701-7. doi: 10.1080/02713680802236185. Curr Eye Res. 2008. PMID: 18696346 Free PMC article. - Immunopathological aspects of age-related macular degeneration.
Patel M, Chan CC. Patel M, et al. Semin Immunopathol. 2008 Apr;30(2):97-110. doi: 10.1007/s00281-008-0112-9. Epub 2008 Feb 26. Semin Immunopathol. 2008. PMID: 18299834 Free PMC article. Review. - Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling.
Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT. Liang KJ, et al. Invest Ophthalmol Vis Sci. 2009 Sep;50(9):4444-51. doi: 10.1167/iovs.08-3357. Epub 2009 May 14. Invest Ophthalmol Vis Sci. 2009. PMID: 19443728 Free PMC article.
References
- la Cour M, Kiilgaard JF, Nissen MH. Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging. 2002;19:101–133. - PubMed
- Espinosa-Heidmann DG, Sall J, Hernandez EP, Cousins SW. Basal laminar deposit formation in APO B100 transgenic mice: complex interactions between dietary fat, blue light, and vitamin E. Invest Ophthalmol Vis Sci. 2004;45:260–266. - PubMed
- Weber BH, Lin B, White K, et al. A mouse model for Sorsby fundus dystrophy. Invest Ophthalmol Vis Sci. 2002;43:2732–2740. - PubMed
- Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Delayed dark-adaptation and lipofuscin accumulation in abcr+/- mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1685–1690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous