RNAi-mediated downregulation of urokinase plasminogen activator receptor and matrix metalloprotease-9 in human breast cancer cells results in decreased tumor invasion, angiogenesis and growth - PubMed (original) (raw)
RNAi-mediated downregulation of urokinase plasminogen activator receptor and matrix metalloprotease-9 in human breast cancer cells results in decreased tumor invasion, angiogenesis and growth
Sateesh Kunigal et al. Int J Cancer. 2007.
Retraction in
- Retraction.
[No authors listed] [No authors listed] Int J Cancer. 2021 Feb 26:10.1002/ijc.33520. doi: 10.1002/ijc.33520. Online ahead of print. Int J Cancer. 2021. PMID: 33908036 Free PMC article. No abstract available.
Abstract
The serine protease urokinase-type plasminogen activator (uPA) plays a significant role in tumor cell invasion and metastasis when bound to its specific receptor, uPAR (also known as CD87). In addition to the uPA-uPAR system, matrix metalloproteinases (MMPs) are involved in tumor cell invasion and metastasis. In this study, we achieved specific inhibition of uPAR and MMP-9 using RNAi technology. We introduced small interfering RNA to downregulate the expression of uPAR and MMP-9 (pUM) in breast cancer cell lines (MDA MB 231 and ZR 75 1). In vitro angiogenesis studies indicated a decrease in the angiogenic potential of the treated cells; in particular, a remarkable decrease was observed in the cells treated with bicistronic construct (pUM) in comparision to the controls. Additionally, bicistronic construct inhibited the formation of capillary-like structures in in vivo models of angiogenesis. Similarly, the invasive potential and migration decreased dramatically when treated with the bicistronic construct as shown by matrigel invasion and migration assays. These results suggest a synergistic effect from the simultaneous downregulation of uPAR and MMP-9. We also assessed the levels of phosphorylated forms of MAPK, ERK and AKT signaling pathway molecules and found reduction in the levels of these molecules in cells treated with the bicistronic construct as compared to the control cells. Furthermore, targeting both uPAR and MMP-9 totally regressed orthotopic breast tumors in nude mice. In conclusion, our results provide evidence that the simultaneous downregulation of uPAR and MMP-9 using RNAi technology may provide an effective tool for breast cancer therapy.
(c) 2007 Wiley-Liss, Inc.
Figures
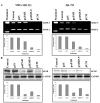
Figure 1. Inhibition of MMP-9 Activity and uPAR Protein Levels by RNA Interference
MDA MB 231 and ZR 751 cells were transfected with CMV promoter-based vectors: control, an empty vector, and a vector encoding either single or bicistronic siRNA for uPAR and MMP-9 (puPAR, pMMP-9, and pUM). (A) MMP-9 activity was measured in conditioned media (40 μg) from SV-, puPAR-, pMMP-9-, and pUM-transfected cells using gelatin zymography. Densitometric analysis was done for MMP-9. (B) Western blot analysis of uPAR protein expression in cell lysates from MDA MB 231 and ZR 751 cells transfected with SV, puPAR, pMMP-9, and pUM. Western blot analysis was performed using an antibody specific for uPAR. GAPDH was simultaneously immunodetected to verify equal loading of cell lysates. Densitometric analysis was done for uPAR expression. The result provided is a representative experiment repeated 3-4 times with concordant results.
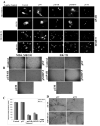
Figure 2. RNA Interference Inhibited uPAR and MMP-9 Immunofluorescence and Tumor-induced Angiogenesis
(A) MDA MB 231 and ZR 751 cells were transfected with SV, puPAR, pMMP-9, and pUM. Control (untransfected) cells were also used. 72 h after transfection, the cells were fixed and stained for uPAR and MMP-9 expression. The cells were mounted using mounting media with 4’,6-diamidino-2-phenylindole (DAPI) to visualize the nucleus. (B) Conditioned media from MDA MB 231 and ZR 751 cells transfected with SV, puPAR, pMMP-9, and pUM were collected. Human microvascular endothelial cells (HMEC) (8×103 cells) were cultured in the conditioned medium collected in 8 well-chambered slides for 12-18 h. After the incubation period, the medium was removed and the cells were stained with HEMA-3 stain and examined under microscope. (C) Quantification of angiogenesis in co-cultures transfected with control, SV, puPAR, pMMP-9, and pUM. Values are mean ± S.D. from four different experiments. (D) Inhibition of tumor angiogenesis by pUM as assessed by mouse dorsal window assay. PV, pre-existing vasculature; TN, tumor-induced vasculature.
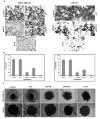
Figure 3. siRNA for uPAR and MMP-9 Inhibits Invasion of MDA MB 231 and ZR 751 cells
MDA MB 231 and ZR 751 cells (1×106) transfected with SV, puPAR, pMMP-9, and pUM were allowed to migrate through matrigel-coated transwell inserts (8-μm pores) for 24 h. (A) The cells that invaded through the matrigel-coated inserts were stained, counted, and photographed under a light microscope at 20X magnification. **(B)**The percentage of invasion was quantified as described in Materials & Methods. Values are mean ± S.D. from five different experiments (p<0.001). (C) We selected MDA MB 231 and ZR 751 cells spheroids that were intact and of approximately the same diameter. Then, the spheroids were transfected with SV, puPAR, pMMP-9 and pUM and incubated for 72 h to allow for migration. Finally, cell migration was observed using a confocal laser-scanning microscope after application of HEMA stain. The result provided is a representative experiment repeated 3-4 times with concordant results.
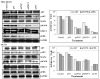
Figure 4. Therapeutic Effect of siRNA for uPAR and MMP-9
MDA MB 231 cells (5-6×106 cells in 100 μL of phosphate-buffered saline or serum-free medium) were injected into the breast pad of nude mice and kept under observation for tumor growth. Tumors were allowed to grow until they reached 5 to 6 mm. At this point, tumors were randomized into several groups receiving treatment with SV, puPAR, pMMP-9, or pUM (150 μg of each vector was injected intratumorally). (A) Animals injected with various constructs. (B) Tumors from these animals were dissected 4 weeks after injection with these constructs. (C) Semiquantification of tumor volume in control, SV, puPAR, pMMP-9, and pUM-treated groups at 4 to 6 weeks after treatment. Data shown are mean ± S.D. values from 5 to 6 animals from each group. (D) Tumor sections stained with hematoxylin and eosin.
Figure 5. siRNA against uPAR and MMP-9 Inhibits the Levels of Phosphorylated ERK, MAPK, and AKT
Western blot analyses of total and phosphorylated forms of p38 MAPK, ERK, and AKT. MDA MB 231 and ZR 751 cells were transfected with SV, puPAR, pMMP-9 and pUM, lysed 72 h later, and subjected to SDS-PAGE and immunoblotting with antibodies for total and phosphorylated forms of MAPK, ERK, and AKT. GADPH antibodies were used to verify that similar amounts of protein were loaded in each lane. Densitometric analysis of phospho- molecules was done. The result provided is of a representative experiment repeated 3-4 times with concordant results.
Similar articles
- Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas.
Lakka SS, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao VH, Sioka C, Rao JS. Lakka SS, et al. J Biol Chem. 2005 Jun 10;280(23):21882-92. doi: 10.1074/jbc.M408520200. Epub 2005 Apr 11. J Biol Chem. 2005. PMID: 15824107 Retracted. - siRNA-mediated simultaneous downregulation of uPA and its receptor inhibits angiogenesis and invasiveness triggering apoptosis in breast cancer cells.
Subramanian R, Gondi CS, Lakka SS, Jutla A, Rao JS. Subramanian R, et al. Int J Oncol. 2006 Apr;28(4):831-9. Int J Oncol. 2006. PMID: 16525631 Free PMC article. - Therapeutic potential of siRNA-mediated targeting of urokinase plasminogen activator, its receptor, and matrix metalloproteinases.
Gondi CS, Rao JS. Gondi CS, et al. Methods Mol Biol. 2009;487:267-81. doi: 10.1007/978-1-60327-547-7_13. Methods Mol Biol. 2009. PMID: 19301652 Free PMC article. Review. - uPAR: An Essential Factor for Tumor Development.
Lv T, Zhao Y, Jiang X, Yuan H, Wang H, Cui X, Xu J, Zhao J, Wang J. Lv T, et al. J Cancer. 2021 Oct 17;12(23):7026-7040. doi: 10.7150/jca.62281. eCollection 2021. J Cancer. 2021. PMID: 34729105 Free PMC article. Review.
Cited by
- Urokinase and its receptors in chronic kidney disease.
Zhang G, Eddy AA. Zhang G, et al. Front Biosci. 2008 May 1;13:5462-78. doi: 10.2741/3093. Front Biosci. 2008. PMID: 18508599 Free PMC article. Review. - Additive impact of HER2-/PTK6-RNAi on interactions with HER3 or IGF-1R leads to reduced breast cancer progression in vivo.
Falkenberg N, Anastasov N, Höfig I, Bashkueva K, Lindner K, Höfler H, Rosemann M, Aubele M. Falkenberg N, et al. Mol Oncol. 2015 Jan;9(1):282-94. doi: 10.1016/j.molonc.2014.08.012. Epub 2014 Sep 6. Mol Oncol. 2015. PMID: 25241146 Free PMC article. - Radiation-inducible silencing of uPA and uPAR in vitro and in vivo in meningioma.
Rao Gogineni V, Kumar Nalla A, Gupta R, Gorantla B, Gujrati M, Dinh DH, Rao JS. Rao Gogineni V, et al. Int J Oncol. 2010 Apr;36(4):809-16. doi: 10.3892/ijo_00000557. Int J Oncol. 2010. PMID: 20198323 Free PMC article. - Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy.
Ushio-Fukai M, Nakamura Y. Ushio-Fukai M, et al. Cancer Lett. 2008 Jul 18;266(1):37-52. doi: 10.1016/j.canlet.2008.02.044. Epub 2008 Apr 10. Cancer Lett. 2008. PMID: 18406051 Free PMC article. Review. - Suppression of Urokinase-Type Plasminogen Activator Receptor by Docosahexaenoic Acid Mediated by Heme Oxygenase-1 in 12-_O_-Tetradecanoylphorbol-13-Acetate-Induced Human Endothelial Cells.
Lian S, Li S, Sah DK, Kim NH, Lakshmanan VK, Jung YD. Lian S, et al. Front Pharmacol. 2020 Nov 26;11:577302. doi: 10.3389/fphar.2020.577302. eCollection 2020. Front Pharmacol. 2020. PMID: 33381031 Free PMC article.
References
- Cottam DW, Rees RC. Regulation of matrix metalloproteinases: their role in tumor invasion and metastasis. Int J Oncol. 1993;2:861–72. - PubMed
- Mignatti P, Robbins E, Rifkin DB. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986;47:487–98. - PubMed
- Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–60. - PubMed
- Dano K, Behrendt N, Brunner N, Ellis V, Ploug M, Pyke C. The urokinse receptor. Protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis. 1994;8:189–203.
- Gondi CS, Lakka SS, Yanamandra N, Siddique K, Dinh DH, Olivero WC, Gujrati M, Rao JS. Expression of antisense uPAR and antisense uPA from a bicistronic adenoviral construct inhibits glioma cell invasion, tumor growth, and angiogenesis. Oncogene. 2003;22:5967–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS47699/NS/NINDS NIH HHS/United States
- R01 NS047699-03/NS/NINDS NIH HHS/United States
- R01 NS057529/NS/NINDS NIH HHS/United States
- R01 CA075557/CA/NCI NIH HHS/United States
- R01 NS057529-01/NS/NINDS NIH HHS/United States
- NS57529/NS/NINDS NIH HHS/United States
- R01 CA075557-09/CA/NCI NIH HHS/United States
- R01 CA092393-04/CA/NCI NIH HHS/United States
- CA 75557/CA/NCI NIH HHS/United States
- CA 92393/CA/NCI NIH HHS/United States
- CA 95058/CA/NCI NIH HHS/United States
- R01 CA116708/CA/NCI NIH HHS/United States
- R01 CA116708-02/CA/NCI NIH HHS/United States
- R01 NS047699/NS/NINDS NIH HHS/United States
- R01 CA095058/CA/NCI NIH HHS/United States
- R01 CA092393/CA/NCI NIH HHS/United States
- CA 116708/CA/NCI NIH HHS/United States
- R01 CA095058-04/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous