A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection - PubMed (original) (raw)
A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection
Xian-Ming Chen et al. J Biol Chem. 2007.
Abstract
Toll-like receptors (TLRs) are important pathogen recognition molecules and are key to epithelial immune responses to microbial infection. However, the molecular mechanisms that regulate TLR expression in epithelia are obscure. Micro-RNAs play important roles in a wide range of biological events through post-transcriptional suppression of target mRNAs. Here we report that human biliary epithelial cells (cholangiocytes) express let-7 family members, micro-RNAs with complementarity to TLR4 mRNA. We found that let-7 regulates TLR4 expression via post-transcriptional suppression in cultured human cholangiocytes. Infection of cultured human cholangiocytes with Cryptosporidium parvum, a parasite that causes intestinal and biliary disease, results in decreased expression of primary let-7i and mature let-7 in a MyD88/NF-kappaB-dependent manner. The decreased let-7 expression is associated with C. parvum-induced up-regulation of TLR4 in infected cells. Moreover, experimentally induced suppression or forced expression of let-7i causes reciprocal alterations in C. parvum-induced TLR4 protein expression, and consequently, infection dynamics of C. parvum in vitro. These results indicate that let-7i regulates TLR4 expression in cholangiocytes and contributes to epithelial immune responses against C. parvum infection. Furthermore, the data raise the possibility that micro-RNA-mediated post-transcriptional pathways may be critical to host-cell regulatory responses to microbial infection in general.
Figures
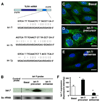
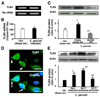
FIGURE 1. Complementarity of let-7 family miRNAs expressed in cholangiocytes to the 3′-UTR of TLR4 mRNA and manipulation of let-7i expression in cultured human cholangiocytes
A, complementarity of let-7 family miRNAs detected in H69 cells to the 3′-UTR of TLR4 mRNA. Using in silico computational target prediction analysis, we identified that the expressed let-7 family members, let-7b, let-7i, and let-7g, have complementarity to the 3′-UTR of TLR4 mRNA. B–F, manipulation of let-7i expression in H69 cells with specific let-7i precursor or antisense oligonucleotide as assessed by Northern blot analysis (B) or by in situ hybridization (C–F). let-7 miRNAs were detected in H69 cells by Northern blot analysis using a probe complementary to let-7i (B). The control probe showed no signal. Whereas cells treated with a let-7i precursor (Ambion) showed an increased signal indicating an increase of let-7, cells treated with a let-7i antisense 2-methoxy oligonucleotide (Ambion) showed a mild decrease of let-7 signal (B). 5S rRNA was probed to confirm equal loading of mRNA. A FITC-tagged antisense oligonucleotide complementary to let-7i was used to visualize let-7 miRNAs. The antisense probe was visualized predominantly in the cytoplasm with a limited detection in the nucleus (C). Furthermore, cells treated with the precursor showed an increased fluorescence (D), whereas cells treated with the antisense oligonucleotide showed a significant decrease of fluorescent signal (E). F, quantitative analysis of fluorescent intensity of the FITC-tagged let-7i antisense oligonucleotide. A total of about 200 cells were randomly selected for each group and each data bar represents mean ± S.D. from three independent experiments. *, p < 0.05, ANOVA versus basal non-transfected control cells (Basal). Bars, 5 µm.
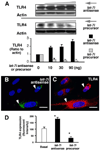
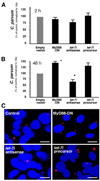
FIGURE 2. let-7i mediates TLR4 expression in cultured cholangiocytes
A, effects of let-7i on TLR4 protein expression by Western analysis. H69 cells were transfected with a let-7i precursor or let-7i antisense 2-methoxy oligonucleotide for 12 h followed by Western blot for TLR4. A dose-dependent increase of TLR4 protein content was detected after treatment with let-7i antisense oligonucleotide. In contrast, overexpression of let-7i with the let-7i precursor decreased TLR4 protein content in a dose-dependent manner. B and C, TLR4 expression in cells transfected with a let-7i antisense oligonucleotide as assessed by immunofluorescence. H69 cells were transfected with a FITC-conjugated antisense oligonucleotide complementary to let-7i for 12 h followed by immunofluorescent staining for TLR4. An increased expression of TLR4 protein (in red, C) was detected in directly transfected cells (arrows, in green, B) compared with non-transfected cells. D, effects of let-7i on TLR4 protein expression by quantitative fluorescent analysis. H69 cells were transfected with either let-7i precursor or let-7i antisense oligonucleotide for 12 h followed by quantitative analysis of immunofluorescent signals for TLR4. A total of about 200 cells were randomly selected for each group and each data bar represents mean ± S.D. from three independent experiments. *, p < 0.05, ANOVA versus with non-transfected control cells (Basal). Bars, 5 µm.
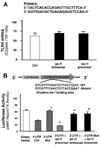
FIGURE 3. let-7i mediates TLR4 protein expression via post-transcriptional suppression
A, effects of let-7i on TLR4 mRNA content. H69 cells were transfected with either let-7i precursor or let-7i antisense oligonucleotide for 12 h followed by quantitative RT-PCR for TLR4 mRNA. Data were normalized to the 18S rRNA level and expressed as copies of TLR4 mRNA/106 copies 18S rRNA. B, targeting of let-7i to the 3′-UTR of TLR4 mRNA. A reporter construct with the potential binding site for let-7 in the 3′-UTR of TLR4 was generated. H69 cells were transiently co-transfected for 24 h with the reporter construct and either let-7i antisense oligonucleotide or let-7i precursor. Luciferase activities were measured and normalized to the control TK Renilla luciferase level. Bars represent the mean ± S.D. from three independent experiments. *, p < 0.05, ANOVA versus cells transfected only with the reporter construct (3′-UTR Ctrl); #, p < 0.05, ANOVA versus cells transfected with the reporter construct plus let-7i precursor (3′ UTR + let-7i precursor).
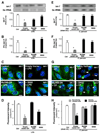
FIGURE 4. LPS stimulation and C. parvum infection decrease let-7i expression in cholangiocytes in a NF-κB dependent manner
A–H, expression of let-7i in cholangiocytes after treatment with LPS (A–D) or infection by C. parvum (E–H). H69 cells, as well as cells stably transfected with a MyD88 functionally deficient dominant negative mutant construct (MyD88-DN) or an empty control vector, were exposed to LPS (100 ng/ml) for 4 h or C. parvum for 12 h followed by Northern blot (A and E), quantitative RT-PCR (B and F) or in situ hybridization (C and G) analysis for let-7i. For Northern blot analysis, 5S rRNA was blotted to confirm that an equal amount of total RNA was used. let-7 signals, detected with the let-7i antisense probe, from three independent experiments were measured using a densitometric analysis and expressed as the ratio to 5S rRNA (A and E). Quantitative RT-PCR analysis was performed with specific primers to let-7i primary transcript and expressed as copies/18S rRNA (B and F). For in situ hybridization analysis, an FITC-tagged antisense probe complementary to let-7i (Ambion) was used to detect let-7 family miRNAs. Cells were also stained with 4′,6-diamidino-2-phenylindole to label the nuclei in blue. Representative confocal images are shown in C and G. C. parvum was stained red using a specific antibody (arrowheads in G). D and H are quantitative analyses of let-7 expression detected with the fluorescently tagged antisense oligonucleotide complementary to let-7i in the cytoplasm of cultured cells by in situ hybridization after treatment with LPS (D) or infection by C. parvum (H), respectively. A total of about 200 cells were randomly selected for each group and each data bar represents mean ± S.D. from three independent experiments. *, p < 0.05, ANOVA versus no-LPS treated control cells (Ctrl, in A, B, and D) or sham infected cells (Sham Inf. Ctrl, in E, F, and H). Bars, 5 µm.
FIGURE 5. let-7i is involved in _C. parvum_-induced up-regulation of TLR4 in cholangiocytes
A and B, TLR4 mRNA expression in cells after exposure to C. parvum for 12 h by RT-PCR (A) and quantitative RT-PCR (B). No significant difference in TLR4 mRNA levels was detected between the sham-infected cells and cells exposed to C. parvum. C, up-regulation of TLR4 protein in cells following C. parvum infection. Whereas a low expression of TLR4 protein was detected in control sham-infected cells, a significant increase of TLR4 protein was found in cells exposed to the parasite. A decreased expression of TLR4 was found in cells transfected with MyD88-DN after exposure to C. parvum. D, up-regulation of TLR4 protein in directly infected cells by immunofluorescent microscopy. TLR4 was stained in green, C. parvum in red, and the nuclei in blue. Increased expression of TLR4 protein (arrowheads) was found only in cells directly infected by the parasite (arrows), not in bystander non-infected cells. E, effects of manipulation of let-7i levels on _C. parvum_-induced TLR4 up-regulation in cholangiocytes as assessed by Western blot and quantitative densitometric analysis. Cells treated with let-7i antisense 2-methoxy oligonucleotide showed a further increase of TLR4 protein content following C. parvum infection compared with infection control cells. In contrast, transfection of cells with the let-7i precursor diminished the _C. parvum_-induced increase of TLR4 protein. *, p < 0.05 compared with sham infected cells (Sham inf. Ctrl). **, p < 0.05, ANOVA versus with cells transfected with the empty vector (C) or cells without oligonucleotide or precursor treatment (E). Bars, 5 µm.
FIGURE 6. let-7i is involved in cholangiocyte immune responses against C. parvum infection in vitro
A, effects of manipulation of let-7i levels on C. parvum attachment and invasion of cholangiocytes in vitro as assessed by quantitative RT-PCR. H69 cells stably transfected with a control empty vector or MyD88-DN construct, as well as let-7i antisense or precursor, were exposed to an equal number of C. parvum for 2 h followed by extensive washing and continued culture. A similar number of parasites was detected as assessed by quantitative RT-PCR in all the cells, including those transfected with let-7i precursor or antisense oligonucleotide, after initial exposure to C. parvum for 2 h. *, p < 0.05, ANOVA versus cells transfected with an empty vector control. B, effects of manipulation of let-7i levels on C. parvum burden in cholangiocytes in vitro 48 h after initial exposure to the parasite by quantitative RT-PCR. A significant increase in parasite number was found in MyD88-DN stably transfected cells and cells transfected with the let-7i precursor 48 h after the initial infection. In contrast, a significantly lower number of parasites were detected in let-7i antisense 2-methoxy oligonucleotide-treated cells. C, effects of manipulation of let-7i levels on C. parvum burden in cholangiocytes in vitro 48 h after initial exposure to the parasite as assessed by immunofluorescent microscopy. C. parvum parasites were stained in red and nuclei in blue. Bars, 5 µm.
Similar articles
- Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB.
Chen XM, O'Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS, Limper AH, LaRusso NF. Chen XM, et al. J Immunol. 2005 Dec 1;175(11):7447-56. doi: 10.4049/jimmunol.175.11.7447. J Immunol. 2005. PMID: 16301652 - Cryptosporidium parvum induces SIRT1 expression in host epithelial cells through downregulating let-7i.
Xie H, Lei N, Gong AY, Chen XM, Hu G. Xie H, et al. Hum Immunol. 2014 Aug;75(8):760-5. doi: 10.1016/j.humimm.2014.05.007. Epub 2014 May 24. Hum Immunol. 2014. PMID: 24862934 Free PMC article. - miR-27b targets KSRP to coordinate TLR4-mediated epithelial defense against Cryptosporidium parvum infection.
Zhou R, Gong AY, Eischeid AN, Chen XM. Zhou R, et al. PLoS Pathog. 2012;8(5):e1002702. doi: 10.1371/journal.ppat.1002702. Epub 2012 May 17. PLoS Pathog. 2012. PMID: 22615562 Free PMC article. - Innate immune responses against Cryptosporidium parvum infection.
McDonald V, Korbel DS, Barakat FM, Choudhry N, Petry F. McDonald V, et al. Parasite Immunol. 2013 Feb;35(2):55-64. doi: 10.1111/pim.12020. Parasite Immunol. 2013. PMID: 23173616 Review. - Regulation of host epithelial responses to Cryptosporidium infection by microRNAs.
Ming Z, Zhou R, Chen XM. Ming Z, et al. Parasite Immunol. 2017 Feb;39(2). doi: 10.1111/pim.12408. Parasite Immunol. 2017. PMID: 27977858 Review.
Cited by
- MicroRNAs and toll-like receptor/interleukin-1 receptor signaling.
Virtue A, Wang H, Yang XF. Virtue A, et al. J Hematol Oncol. 2012 Oct 18;5:66. doi: 10.1186/1756-8722-5-66. J Hematol Oncol. 2012. PMID: 23078795 Free PMC article. Review. - microRNAs associated with the pathogenesis and their role in regulating various signaling pathways during Mycobacterium tuberculosis infection.
Davuluri KS, Chauhan DS. Davuluri KS, et al. Front Cell Infect Microbiol. 2022 Oct 27;12:1009901. doi: 10.3389/fcimb.2022.1009901. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389170 Free PMC article. Review. - Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family.
Takahashi M, Sung B, Shen Y, Hur K, Link A, Boland CR, Aggarwal BB, Goel A. Takahashi M, et al. Carcinogenesis. 2012 Dec;33(12):2441-9. doi: 10.1093/carcin/bgs286. Epub 2012 Sep 15. Carcinogenesis. 2012. PMID: 22983985 Free PMC article. - The Emerging Role of microRNA in Periodontitis: Pathophysiology, Clinical Potential and Future Molecular Perspectives.
Santonocito S, Polizzi A, Palazzo G, Isola G. Santonocito S, et al. Int J Mol Sci. 2021 May 21;22(11):5456. doi: 10.3390/ijms22115456. Int J Mol Sci. 2021. PMID: 34064286 Free PMC article. Review. - Mini but mighty: microRNAs in the pathobiology of periodontal disease.
Kebschull M, Papapanou PN. Kebschull M, et al. Periodontol 2000. 2015 Oct;69(1):201-20. doi: 10.1111/prd.12095. Periodontol 2000. 2015. PMID: 26252410 Free PMC article. Review.
References
- Takeda K, Kaisho T, Akira S. Annu. Rev. Immunol. 2003;21:335–376. - PubMed
- Akira S, Takeda K. Nat. Rev. Immunol. 2004;4:499–511. - PubMed
- Modlin RL, Cheng G. Nat. Med. 2004;10:1173–1174. - PubMed
- Strober W. Nat. Med. 2004;10:898–900. - PubMed
- Campos MA, Campos MA, Closel M, Valente EP, Cardoso JE, Akira S, Alvarez-Leite JI, Ropert C, Gazzinelli RT. J. Immunol. 2004;172:1711–1718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK24031/DK/NIDDK NIH HHS/United States
- R01 DK057993/DK/NIDDK NIH HHS/United States
- R01 AI071321/AI/NIAID NIH HHS/United States
- DK57993/DK/NIDDK NIH HHS/United States
- R01 DK024031/DK/NIDDK NIH HHS/United States
- AI071321/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases