Kinase activity is not required for alphaCaMKII-dependent presynaptic plasticity at CA3-CA1 synapses - PubMed (original) (raw)
Kinase activity is not required for alphaCaMKII-dependent presynaptic plasticity at CA3-CA1 synapses
Mohammad Reza Hojjati et al. Nat Neurosci. 2007 Sep.
Abstract
Using targeted mouse mutants and pharmacologic inhibition of alphaCaMKII, we demonstrate that the alphaCaMKII protein, but not its activation, autophosphorylation or its ability to phosphorylate synapsin I, is required for normal short-term presynaptic plasticity. Furthermore, alphaCaMKII regulates the number of docked vesicles independent of its ability to be activated. These results indicate that alphaCaMKII has a nonenzymatic role in short-term presynaptic plasticity at hippocampal CA3-CA1 synapses.
Figures
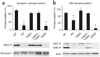
Figure 1
Phosphorylation of synapsin I and CaMKII-T286/T287 in synaptosomes obtained from αCaMKII mutants. (a) Phosphorylation of synapsin I S603 was not affected by impaired αCaMKII autophosphorylation, but required αCaMKII protein and its activation by Ca2+/calmodulin. (b) Phosphorylation of αCaMKII-T286 and βCaMKII-T287 was absent in αCaMKII-T305D mice. Graph represents data from βCaMKII-T287 only. Error bars indicate s.e.m. Each sample contains pooled fractions from four independent isolations.
Figure 2
Presynaptic short-term plasticity requires αCaMKII protein, but not its autophosphorylation, activation or activity. (a–c) Increased synaptic augmentation in αCaMKII-KO mutant mice was not caused by the lack of CaMKII kinase activity. fEPSP responses (normalized to pretetanus baseline) of CaMKII-KO (a) and CaMKII-T305D (b) mice were recorded at the indicated time after a 10 theta-burst tetanus. Traces are from baseline response (gray) and the response 5 s post-tetanization (black). Augmentation summary of responses obtained 5 s post-tetanus normalized to baseline is shown in c. Black bars represent mutants or drug-treated slices, white bars represent control slices as indicated. (d–f). Decreased synaptic fatigue during repetitive stimulation in αCaMKII-KO mice was not caused by the lack of αCaMKII kinase activity. (d,e) fEPSP responses (normalized against baseline) of CaMKII-KO (d) and CaMKII-T305D (e) mice were recorded during a 10-Hz tetanus. Only the first and even numbered stimuli are shown for clarity. Traces are from wild-type (gray) and αCaMKII-KO slices (black) recorded from stimulus number 21–30. Depletion summary of the last (100) stimulus of the 10-Hz train is shown in f. Black bars represent mutant or drug-treated slices, normalized against the controls as indicated (white bars, set at 100%). Numbers between brackets indicate the number of slices. Error bars indicate s.e.m.
Figure 3
αCaMKII protein regulates the number of docked vesicles. (a) Quantitative electron microscopy of asymmetric synapses on dendritic spines of CA1 pyramidal neurons showed a 20% increase in the number of docked vesicles in αCaMKII-KO mice. (b) Decreasing the depletion rate by lowering extracellular calcium reversed the phenotype of the αCaMKII-KO mice during repetitive stimulation. Error bars indicate s.e.m.
Similar articles
- Impaired hippocampal synaptic transmission and plasticity in mice lacking fibroblast growth factor 14.
Xiao M, Xu L, Laezza F, Yamada K, Feng S, Ornitz DM. Xiao M, et al. Mol Cell Neurosci. 2007 Mar;34(3):366-77. doi: 10.1016/j.mcn.2006.11.020. Epub 2007 Jan 8. Mol Cell Neurosci. 2007. PMID: 17208450 - Autophosphorylation of alphaCaMKII downregulates excitability of CA1 pyramidal neurons following synaptic stimulation.
Sametsky EA, Disterhoft JF, Ohno M. Sametsky EA, et al. Neurobiol Learn Mem. 2009 Jul;92(1):120-3. doi: 10.1016/j.nlm.2009.02.006. Epub 2009 Feb 24. Neurobiol Learn Mem. 2009. PMID: 19245842 Free PMC article. - Role of GluA3 AMPA Receptor Subunits in the Presynaptic and Postsynaptic Maturation of Synaptic Transmission and Plasticity of Endbulb-Bushy Cell Synapses in the Cochlear Nucleus.
Antunes FM, Rubio ME, Kandler K. Antunes FM, et al. J Neurosci. 2020 Mar 18;40(12):2471-2484. doi: 10.1523/JNEUROSCI.2573-19.2020. Epub 2020 Feb 12. J Neurosci. 2020. PMID: 32051325 Free PMC article. - Transcellular Nanoalignment of Synaptic Function.
Biederer T, Kaeser PS, Blanpied TA. Biederer T, et al. Neuron. 2017 Nov 1;96(3):680-696. doi: 10.1016/j.neuron.2017.10.006. Neuron. 2017. PMID: 29096080 Free PMC article. Review. - Participation of CaMKII in neuronal plasticity and memory formation.
Cammarota M, Bevilaqua LR, Viola H, Kerr DS, Reichmann B, Teixeira V, Bulla M, Izquierdo I, Medina JH. Cammarota M, et al. Cell Mol Neurobiol. 2002 Jun;22(3):259-67. doi: 10.1023/a:1020763716886. Cell Mol Neurobiol. 2002. PMID: 12469869 Review.
Cited by
- Analog Signaling With the "Digital" Molecular Switch CaMKII.
Clarke SE. Clarke SE. Front Comput Neurosci. 2018 Nov 22;12:92. doi: 10.3389/fncom.2018.00092. eCollection 2018. Front Comput Neurosci. 2018. PMID: 30524260 Free PMC article. - Drosophila rolling blackout displays lipase domain-dependent and -independent endocytic functions downstream of dynamin.
Vijayakrishnan N, Phillips SE, Broadie K. Vijayakrishnan N, et al. Traffic. 2010 Dec;11(12):1567-78. doi: 10.1111/j.1600-0854.2010.01117.x. Epub 2010 Oct 1. Traffic. 2010. PMID: 21029287 Free PMC article. - In vivo amphetamine action is contingent on αCaMKII.
Steinkellner T, Mus L, Eisenrauch B, Constantinescu A, Leo D, Konrad L, Rickhag M, Sørensen G, Efimova EV, Kong E, Willeit M, Sotnikova TD, Kudlacek O, Gether U, Freissmuth M, Pollak DD, Gainetdinov RR, Sitte HH. Steinkellner T, et al. Neuropsychopharmacology. 2014 Oct;39(11):2681-93. doi: 10.1038/npp.2014.124. Epub 2014 May 29. Neuropsychopharmacology. 2014. PMID: 24871545 Free PMC article. - Long-term potentiation can be induced in the CA1 region of hippocampus in the absence of αCaMKII T286-autophosphorylation.
Villers A, Giese KP, Ris L. Villers A, et al. Learn Mem. 2014 Oct 16;21(11):616-26. doi: 10.1101/lm.035972.114. Print 2014 Nov. Learn Mem. 2014. PMID: 25322797 Free PMC article. - Mechanisms of CaMKII action in long-term potentiation.
Lisman J, Yasuda R, Raghavachari S. Lisman J, et al. Nat Rev Neurosci. 2012 Feb 15;13(3):169-82. doi: 10.1038/nrn3192. Nat Rev Neurosci. 2012. PMID: 22334212 Free PMC article. Review.
References
- Bennett MK, Erondu NE, Kennedy MB. J. Biol. Chem. 1983;258:12735–12744. - PubMed
- Benfenati F, et al. Nature. 1992;359:417–420. - PubMed
- Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. Neuron. 1995;14:591–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS047466/NS/NINDS NIH HHS/United States
- P30 NS057098/NS/NINDS NIH HHS/United States
- P30 HD038985/HD/NICHD NIH HHS/United States
- R01 NS040593/NS/NINDS NIH HHS/United States
- P30-HD38985/HD/NICHD NIH HHS/United States
- P30-NS47466/NS/NINDS NIH HHS/United States
- P30-NS57098/NS/NINDS NIH HHS/United States
- R01 NS040593-09/NS/NINDS NIH HHS/United States
- G0400983/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous