A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions - PubMed (original) (raw)
A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions
Andrei I Ivanov et al. PLoS One. 2007.
Abstract
The integrity and function of the epithelial barrier is dependent on the apical junctional complex (AJC) composed of tight and adherens junctions and regulated by the underlying actin filaments. A major F-actin motor, myosin II, was previously implicated in regulation of the AJC, however direct evidence of the involvement of myosin II in AJC dynamics are lacking and the molecular identity of the myosin II motor that regulates formation and disassembly of apical junctions in mammalian epithelia is unknown. We investigated the role of nonmuscle myosin II (NMMII) heavy chain isoforms, A, B, and C in regulation of epithelial AJC dynamics and function. Expression of the three NMMII isoforms was observed in model intestinal epithelial cell lines, where all isoforms accumulated within the perijunctional F-actin belt. siRNA-mediated downregulation of NMMIIA, but not NMMIIB or NMMIIC expression in SK-CO15 colonic epithelial cells resulted in profound changes of cell morphology and cell-cell adhesions. These changes included acquisition of a fibroblast-like cell shape, defective paracellular barrier, and substantial attenuation of the assembly and disassembly of both adherens and tight junctions. Impaired assembly of the AJC observed after NMMIIA knock-down involved dramatic disorganization of perijunctional actin filaments. These findings provide the first direct non-pharmacological evidence of myosin II-dependent regulation of AJC dynamics in mammalian epithelia and highlight a unique role of NMMIIA in junctional biogenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
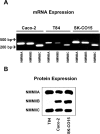
Figure 1. Expression of nonmuscle myosin II isoforms in cultured human intestinal epithelial cells.
mRNA (A) and protein (B) expression of NMMIIA, NMMIIB and NMMIIC was analyzed in different intestinal epithelial cell lines using isoform-specific primers and polyclonal antibodies. NMMIIA and NMMIIC are expresses in all studied epithelial cells lines, whereas protein expression of NMMIIB is abundant in SK-CO15 and Caco-2 cells but is undetectable by Western blotting in T84 colonic epithelial cells.
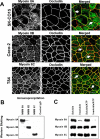
Figure 2. Localization and biochemical properties of different NMMII isoforms in cultured human intestinal epithelial cells.
(A) Confluent SK-CO15, Caco-2 and T84 cell monolayers were double-immunolabeled for either NMMIIA, NMMIIB, or NMMIIC (green) and the TJ protein, occludin (red). All three NMMII isoforms colocalize with occludin at the mature AJC (arrows). Bar, 10 µm. (B). NMMIIA, NMMIIB, and NMMIIC were immunoprecipitated from SK-CO15 cell lysates using isoform-specific polyclonal antibodies. Little or no cross-precipitation of the different NMMII isoforms is observed. (C) Detergent solubility of different NMMII isoforms was analyzed using TX-100 fractionation of SK-CO15 cell monolayers. The majority of NMMIIA is Triton-soluble, especially in the presence of 1 mM ATP, whereas significant Triton-insoluble fraction is characteristics to NMMIIB and NMMIIC.
Figure 3. Downregulation of the NMMIIA expression alters epithelial cell shape and attenuates development of the paracellular barrier.
(A) Western blots of SK-CO15 cell lysates prepared 3 days after transfection show selective downregulation of protein expression of NMMIIA. NMMIIB, and NMMIIC by two different siRNA duplexes, each specific for the NMMII isoform. (B) siRNA-mediated knock-down of NMMIIA but not NMMIIB or NMMIIC causes dramatic changes from an orthogonal epithelial to a protrusive fibroblast-like shape in low-density colonies of SK-CO15 cells. (C) siRNA -mediated knock-down of NMMIIA but not NMMIIB or NMMIIC significantly attenuates the increase in TEER in confluent SK-CO15 cell monolayers when compared to the control (cyclophilin) siRNA-transfected cells (*p<0.05; n = 4).
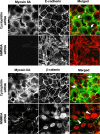
Figure 4. Downregulation of the NMMIIA expression impedes reformation of initial adherens-like junctions.
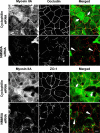
SK-CO15 cells were transfected with either control (cyclophilin) or NMMII isoform specific siRNAs and on day 3 post-transfection were subjected to overnight calcium depletion in order to disrupt cell-cell adhesion. Reformation of initial adherens-like junctions was triggered by transferring cells for 1 h into the HCM. Control cells show rapid accumulation of E-cadherin and β-catenin (red) in areas of cell-cell contacts (arrows). In contrast, the majority of E-cadherin remains in the cytosol and β-catenin localizes in the nuclei in NMMIIA-depleted cells (arrowheads). Bar, 10 µm.
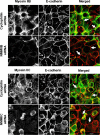
Figure 5. siRNA-mediated knock-down of either NMMIIB or NMMIIC has no effect on reformation of initial adherens-like junctions.
Control, NMMIIB or NMMIIC siRNA-transfected SK-CO15 monolayers were subjected to overnight calcium depletion to disrupt cell-cell adhesion. Reformation of the initial adherens-like junctions was triggered by transferring cells for 1 h into the HCM. Similarly to control cells, NMMIIB and NMMIIC knock-down rapidly translocate E-cadherin (red) to areas of cell-cell contacts (arrows). Bar, 10 µm.
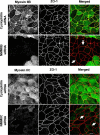
Figure 6. siRNA-mediated depletion of NMMIIA attenuates the development of tight junctions.
SK-CO15 cells were transfected with either control (cyclophilin) or NMMII isoform specific siRNAs and on day 3 post-transfection were subjected to overnight calcium depletion in order to disrupt cell-cell adhesion. Reassembly of TJs in control and NMMIIA-deficient cells was investigated after 5 h of calcium repletion by monitoring the formation of characteristic ‘chicken wire’ labeling pattern of the TJ proteins occludin and ZO-1 (red). Control SK-CO15 cell monolayers show almost complete restoration of normal localization of occludin and ZO-1 at TJs (arrows). In contrast, occludin and ZO-1 labeling demonstrates abnormal discontinuous pattern at TJs in NMMIIA-deficient cells (arrowheads). Bar, 10 µm.
Figure 7. Downregulation of either NMMIIB or NMMIIC has no effect on reformation of epithelial TJs.
Control, NMMIIB or NMMIIC siRNA-transfected SK-CO15 monolayers were subjected to overnight calcium depletion to disrupt cell-cell adhesion. Reformation of TJs was triggered by transferring cells for 5 h into the HCM. Similarly to control cells, NMMIIB and NMMIIC-deficient cell monolayers rapidly restore normal junctional labeling pattern for ZO-1 (arrows). Bar, 10 µm.
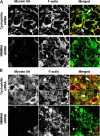
Figure 8. siRNA knock-down of NMMIIA causes disorganization of F-actin cytoskeleton.
Calcium-depleted control and NMMIIA-deficient SK-CO15 cell monolayers were transferred into the HCM for 0.5 h (A) and 5 h (B) to trigger junctional reassembly. Organization of their actin filaments was visualized using fluorescently labeled phalloidin. At the early time of calcium repletion, prominent radial F-actin cables can be seen in lamellipodia of spreading/contacting control cells (A, arrows). At a later time, control cells show circumferential apical F-actin bundles (B, arrowheads). Neither structure is formed in NMMIIA-deficient cells which show diffuse (A) or abnormally aggregated (B, stars) F-actin. Bar, 10 µm.
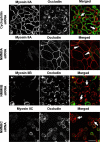
Figure 9. siRNA-mediated knock-down of NMMIIA selectively attenuates disassembly of the AJC in calcium-depleted cells.
Control, NMMIIA, NMMIIB, and NMMIIC-deficient SK-CO15 monolayers were incubated for 1 h in the LCM-EGTA, and the integrity of their AJC was analyzed by immunolabeling for occludin (red). Calcium depletion causes rapid disruption of the AJC and accumulation of occludin in cytosolic ring-like structures in control, NMMIIB and NMMIIC-deficient cells (arrows). In contrast, the majority of occludin-labeled TJs remained intact in cells monolayers subjected to NMMIIA knock-down (arrowheads). Bar, 20 µm.
Figure 10. siRNA-mediated knock-down of NMMIIA inhibits cell contractility triggered by calcium-depletion.
Control, NMMIIA, NMMIIB, and NMMIIC-deficient SK-CO15 monolayers were subjected to 1 h calcium depletion, and their overall cell shape was analyzed by F-actin labeling. Calcium depletion causes rapid rounding of control, NMMIIB, and NMMIIC-deficient cells (arrows). In contrast, cell rounding was significantly attenuated in cells monolayers subjected to NMMIIA knock-down. Bar, 20 µm.
Similar articles
- Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex.
Ivanov AI, McCall IC, Parkos CA, Nusrat A. Ivanov AI, et al. Mol Biol Cell. 2004 Jun;15(6):2639-51. doi: 10.1091/mbc.e04-02-0163. Epub 2004 Mar 26. Mol Biol Cell. 2004. PMID: 15047870 Free PMC article. - Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex.
Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Ivanov AI, et al. Mol Biol Cell. 2005 Jun;16(6):2636-50. doi: 10.1091/mbc.e05-01-0043. Epub 2005 Mar 30. Mol Biol Cell. 2005. PMID: 15800060 Free PMC article. - Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions.
Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Samarin SN, et al. Mol Biol Cell. 2007 Sep;18(9):3429-39. doi: 10.1091/mbc.e07-04-0315. Epub 2007 Jun 27. Mol Biol Cell. 2007. PMID: 17596509 Free PMC article. - Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers.
Ivanov AI, Nusrat A, Parkos CA. Ivanov AI, et al. Bioessays. 2005 Apr;27(4):356-65. doi: 10.1002/bies.20203. Bioessays. 2005. PMID: 15770686 Review. - Unique and redundant functions of cytoplasmic actins and nonmuscle myosin II isoforms at epithelial junctions.
Ivanov AI, Lechuga S, Marino-Melendez A, Naydenov NG. Ivanov AI, et al. Ann N Y Acad Sci. 2022 Sep;1515(1):61-74. doi: 10.1111/nyas.14808. Epub 2022 Jun 7. Ann N Y Acad Sci. 2022. PMID: 35673768 Free PMC article. Review.
Cited by
- Claudin-23 reshapes epithelial tight junction architecture to regulate barrier function.
Raya-Sandino A, Lozada-Soto KM, Rajagopal N, Garcia-Hernandez V, Luissint AC, Brazil JC, Cui G, Koval M, Parkos CA, Nangia S, Nusrat A. Raya-Sandino A, et al. Nat Commun. 2023 Oct 5;14(1):6214. doi: 10.1038/s41467-023-41999-9. Nat Commun. 2023. PMID: 37798277 Free PMC article. - Role of Nonmuscle Myosin II in Migration of Wharton's Jelly-Derived Mesenchymal Stem Cells.
Arora S, Saha S, Roy S, Das M, Jana SS, Ta M. Arora S, et al. Stem Cells Dev. 2015 Sep 1;24(17):2065-77. doi: 10.1089/scd.2015.0095. Epub 2015 Jun 4. Stem Cells Dev. 2015. PMID: 25923805 Free PMC article. - CRB3A Controls the Morphology and Cohesion of Cancer Cells through Ehm2/p114RhoGEF-Dependent Signaling.
Loie E, Charrier LE, Sollier K, Masson JY, Laprise P. Loie E, et al. Mol Cell Biol. 2015 Oct;35(19):3423-35. doi: 10.1128/MCB.00673-15. Epub 2015 Jul 27. Mol Cell Biol. 2015. PMID: 26217016 Free PMC article. - Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis.
Naydenov NG, Feygin A, Wang D, Kuemmerle JF, Harris G, Conti MA, Adelstein RS, Ivanov AI. Naydenov NG, et al. Sci Rep. 2016 Apr 11;6:24161. doi: 10.1038/srep24161. Sci Rep. 2016. PMID: 27063635 Free PMC article. - Virulence factors impair epithelial junctions during bacterial infection.
Zheng M, Sun S, Zhou J, Liu M. Zheng M, et al. J Clin Lab Anal. 2021 Feb;35(2):e23627. doi: 10.1002/jcla.23627. Epub 2020 Oct 17. J Clin Lab Anal. 2021. PMID: 33070380 Free PMC article. Review.
References
- Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol. 2004;16:140–145. - PubMed
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. - PubMed
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. - PubMed
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. - PubMed
- Blaschuk OW, Rowlands TM. Plasma membrane components of adherens junctions. Mol Membr Biol. 2002;19:75–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 DK055679/DK/NIDDK NIH HHS/United States
- HL72124/HL/NHLBI NIH HHS/United States
- DK 55679/DK/NIDDK NIH HHS/United States
- DK 61379/DK/NIDDK NIH HHS/United States
- R01 DK072564/DK/NIDDK NIH HHS/United States
- DK 72564/DK/NIDDK NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- R01 DK055679/DK/NIDDK NIH HHS/United States
- DK 59888/DK/NIDDK NIH HHS/United States
- R01 DK061379/DK/NIDDK NIH HHS/United States
- R01 HL072124/HL/NHLBI NIH HHS/United States
- R01 DK059888/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources