Intense sweetness surpasses cocaine reward - PubMed (original) (raw)
Intense sweetness surpasses cocaine reward
Magalie Lenoir et al. PLoS One. 2007.
Abstract
Background: Refined sugars (e.g., sucrose, fructose) were absent in the diet of most people until very recently in human history. Today overconsumption of diets rich in sugars contributes together with other factors to drive the current obesity epidemic. Overconsumption of sugar-dense foods or beverages is initially motivated by the pleasure of sweet taste and is often compared to drug addiction. Though there are many biological commonalities between sweetened diets and drugs of abuse, the addictive potential of the former relative to the latter is currently unknown.
Methodology/principal findings: Here we report that when rats were allowed to choose mutually-exclusively between water sweetened with saccharin-an intense calorie-free sweetener-and intravenous cocaine-a highly addictive and harmful substance-the large majority of animals (94%) preferred the sweet taste of saccharin. The preference for saccharin was not attributable to its unnatural ability to induce sweetness without calories because the same preference was also observed with sucrose, a natural sugar. Finally, the preference for saccharin was not surmountable by increasing doses of cocaine and was observed despite either cocaine intoxication, sensitization or intake escalation-the latter being a hallmark of drug addiction.
Conclusions: Our findings clearly demonstrate that intense sweetness can surpass cocaine reward, even in drug-sensitized and -addicted individuals. We speculate that the addictive potential of intense sweetness results from an inborn hypersensitivity to sweet tastants. In most mammals, including rats and humans, sweet receptors evolved in ancestral environments poor in sugars and are thus not adapted to high concentrations of sweet tastants. The supranormal stimulation of these receptors by sugar-rich diets, such as those now widely available in modern societies, would generate a supranormal reward signal in the brain, with the potential to override self-control mechanisms and thus to lead to addiction.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
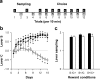
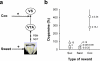
Figure 1. Choice between saccharin and cocaine.
a, Schematic representation of the choice procedure. Each choice session was constituted of 12 discrete trials, spaced by 10 min, and divided into two successive phases, sampling (4 trials) followed by choice (8 trials). S, saccharin-associated lever; C, cocaine-associated lever. b, Choice between levers C and S (mean±SEM) across reward conditions and as a function of time (open circle: S-/C+ condition; closed triangle: S+/C- condition; closed circle: S+/C+ condition). The horizontal gray line at 0 indicates the indifference level. Values above 0 indicate a preference for lever S while values below 0 indicate a preference for lever C. *, different from the indifference level (P<0.05, _t_-test). c, Sampling (mean±SEM of the last 3 days) of lever S (black bars) and lever C (white bars) across reward conditions. *, different from lever S (P<0.05, Fisher's LSD test after a two-way analysis of variance).
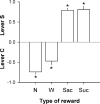
Figure 2. Choice between lever C and no fluid (N), water (W), saccharin (Sac, 0.2%) or sucrose (Suc, 4%).
The horizontal gray line at 0 indicates the indifference level. Values above 0 indicate a preference for lever S while values below 0 indicate a preference for lever C. *, different from the indifference level (P<0.05, _t_-test). Each reward type (N, W, Sac and Suc) was tested at least 5 times in a row until stabilization of behavior. Bars represent the means (±SEM) of the last 3 stable days. The first 3 reward conditions (N, W and Sac in this order) were tested in the same group of animals (N = 10) while the sucrose condition was tested in a separate group (N = 10).
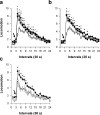
Figure 3. Cocaine-induced locomotion in rats tested under.
a, the S-/C+ condition, or b, the S+/C+ condition. Locomotion (i.e., mean number of cage crossings±SEM) was measured during 10 min after the first cocaine self-injection (0.25 mg, i.v.) of the day (open triangle: day 1; closed circle: day 5; closed square: day 15). *, day 5 different from day 1; °, day 15 different from day 1 (P<0.05, Fisher's LSD test). c, Effects of the first cocaine self-injection in rats initially trained under the S+/C- condition and tested for the first time under the S+/C+ condition on day 16. These effects (open square) were compared to the effects of cocaine on day 15 in rats initially trained under the S+/C+ (closed square). *, P<0.05, Fisher's LSD test. The arrow in all graphs indicates the intravenous injection of cocaine.
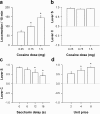
Figure 4. Pharmacological and economic determinants of cocaine choice.
a, Cocaine-induced locomotion as a function of dose. Locomotion (i.e., mean number of cage crossings±SEM) was measured during 10 min after the first cocaine self-injection of the first day of each dose substitution. b, Choice between levers C and S (mean±SEM) as a function of dose. c, Choice between levers C and S (mean±SEM) as a function of delay between response and saccharin delivery. *, different from the shortest delay (P<0.05, Fisher's LSD test after one-way ANOVA). d, Choice between levers C and S (mean±SEM) as a function of reward price. *, different from the lowest price (P<0.05, Fisher's LSD test after one-way ANOVA). The values of each variable (dose, delay and price) were tested at least 5 times in a row until stabilization of behavior. Bars represent the means of the last 3 stable days.
Figure 5. Choice between saccharin and cocaine as a function of drug history.
a, Reversal of preference in rats which had acquired a preference for lever C under the S-/C+ condition. The first 3 days (-3 to -1) correspond to baseline choice under the S-/C+ condition. The next 10 days correspond to choice after the shift to the S+/C+ condition. b, Choice between levers C and S (mean±SEM) after cocaine intake escalation. Insert: Choice between levers C and S as a function of the dose. c, Cocaine self-injections (mean±SEM) during the hour preceding choice in the modified discrete-trials choice procedure. d, Choice between levers C and S (mean±SEM) during cocaine intoxication. e, Representative individual distributions of cocaine rewards (downward ticks) or saccharin rewards (upward ticks) within the last testing session. The vertical dashed line separates the 1-hour exclusive access to cocaine self-administration (C+ only) from the subsequent 8 discrete choices (S+/C+ condition). *, different from the indifference level (P<0.05, _t_-test); +, different from the lowest dose (P<0.05, Fisher's LSD test after one-way ANOVA).
Figure 6. Effects of sucrose, saccharin or cocaine consumption on ventral striatal dopamine levels.
a, Consumption of sweet solutions turns on midbrain dopamine cells that projects to the ventral striatum, possibly through a short, two-relay circuit in the brain stem . In contrast, cocaine directly increases dopamine levels in the ventral striatum by blocking dopamine uptake. The symbol+indicates pharmacological or sensory stimulation and the symbol x, intermediate synapses. NST, nucleus of the solitary tract; PBN, parabrachial nucleus; VS, ventral striatum; VTA, ventral tegmental area. b, Mean (±SEM) levels of extra-cellular dopamine in the ventral striatum (expressed as percent change from baseline) during sucrose, saccharin or cocaine intake. These results are based on a meta-analysis of the literature (see Materials and Methods). Values that appear on the right of symbols represent sucrose or saccharin concentrations (in %) and cocaine doses (in mg/kg).
Similar articles
- Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues.
Madsen HB, Ahmed SH. Madsen HB, et al. Addict Biol. 2015 May;20(3):433-44. doi: 10.1111/adb.12134. Epub 2014 Mar 7. Addict Biol. 2015. PMID: 24602027 - Overexpression of DeltaFosB is associated with attenuated cocaine-induced suppression of saccharin intake in mice.
Freet CS, Steffen C, Nestler EJ, Grigson PS. Freet CS, et al. Behav Neurosci. 2009 Apr;123(2):397-407. doi: 10.1037/a0015033. Behav Neurosci. 2009. PMID: 19331462 Free PMC article. - Ceftriaxone attenuates acquisition and facilitates extinction of cocaine-induced suppression of saccharin intake in C57BL/6J mice.
Freet CS, Lawrence AL. Freet CS, et al. Physiol Behav. 2015 Oct 1;149:174-80. doi: 10.1016/j.physbeh.2015.06.009. Epub 2015 Jun 9. Physiol Behav. 2015. PMID: 26066719 Free PMC article. - Sugar addiction: pushing the drug-sugar analogy to the limit.
Ahmed SH, Guillem K, Vandaele Y. Ahmed SH, et al. Curr Opin Clin Nutr Metab Care. 2013 Jul;16(4):434-9. doi: 10.1097/MCO.0b013e328361c8b8. Curr Opin Clin Nutr Metab Care. 2013. PMID: 23719144 Review. - Sugars and Sweet Taste: Addictive or Rewarding?
Greenberg D, St Peter JV. Greenberg D, et al. Int J Environ Res Public Health. 2021 Sep 17;18(18):9791. doi: 10.3390/ijerph18189791. Int J Environ Res Public Health. 2021. PMID: 34574716 Free PMC article. Review.
Cited by
- Extended heroin access increases heroin choices over a potent nondrug alternative.
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Lenoir M, et al. Neuropsychopharmacology. 2013 Jun;38(7):1209-20. doi: 10.1038/npp.2013.17. Epub 2013 Jan 15. Neuropsychopharmacology. 2013. PMID: 23322185 Free PMC article. - Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration.
Banks ML, Negus SS. Banks ML, et al. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. Epub 2012 Nov 28. Adv Pharmacol Sci. 2012. PMID: 23243420 Free PMC article. - Obesity and addiction: neurobiological overlaps.
Volkow ND, Wang GJ, Tomasi D, Baler RD. Volkow ND, et al. Obes Rev. 2013 Jan;14(1):2-18. doi: 10.1111/j.1467-789X.2012.01031.x. Epub 2012 Sep 27. Obes Rev. 2013. PMID: 23016694 Free PMC article. Review. - Fos-expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats.
Kane L, Venniro M, Quintana-Feliciano R, Madangopal R, Rubio FJ, Bossert JM, Caprioli D, Shaham Y, Hope BT, Warren BL. Kane L, et al. Addict Biol. 2021 May;26(3):e12943. doi: 10.1111/adb.12943. Epub 2020 Jul 19. Addict Biol. 2021. PMID: 32683756 Free PMC article. - Role of nucleus accumbens core but not shell in incubation of methamphetamine craving after voluntary abstinence.
Rossi LM, Reverte I, Ragozzino D, Badiani A, Venniro M, Caprioli D. Rossi LM, et al. Neuropsychopharmacology. 2020 Jan;45(2):256-265. doi: 10.1038/s41386-019-0479-4. Epub 2019 Aug 18. Neuropsychopharmacology. 2020. PMID: 31422417 Free PMC article.
References
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–94. - PubMed
- Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–64. - PubMed
- Steiner JE. Human facial expressions in response to taste and smell stimulation. Adv Child Dev Behav. 1979;13:257–95. - PubMed
- Drewnowski A. Taste preferences and food intake. Annu Rev Nutr. 1997;17:237–53. - PubMed
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical